3 Minerals
By the end of this chapter, students should be able to:
- Define mineral.
- Describe the basic structure of the atom.
- Derive basic atomic information from the Periodic Table of Elements.
- Describe chemical bonding related to minerals.
- Describe the main ways minerals form.
- Describe the silicon-oxygen tetrahedron and how it forms common silicate minerals.
- List common non-silicate minerals in oxide, sulfide, sulfate, and carbonate groups.
- Identify minerals using physical properties and identification tables.
The term “minerals” as used in nutrition labels and pharmaceutical products is not the same as a mineral in a geological sense. In geology, the classic definition of a mineral is: 1) naturally occurring, 2) inorganic, 3) solid at room temperature, 4) regular crystal structure, and 5) defined chemical composition. Some natural substances technically should not be considered minerals, but are included by exception. For example, water and mercury are liquid at room temperature. Both are considered minerals because they were classified before the room-temperature rule was accepted as part of the definition. Calcite is quite often formed by organic processes, but is considered a mineral because it is widely found and geologically important. Because of these discrepancies, the International Mineralogical Association in 1985 amended the definition to: “A mineral is an element or chemical compound that is normally crystalline and that has been formed as a result of geological processes.” This means that the calcite in the shell of a clam is not considered a mineral. But once that clam shell undergoes burial, diagenesis, or other geological processes, then the calcite is considered a mineral. Typically, substances like coal, pearl, opal, or obsidian that do not fit the definition of mineral are called mineraloids.
A rock is a substance that contains one or more minerals or mineraloids. As is discussed in later chapters, there are three types of rocks composed of minerals: igneous (rocks crystallizing from molten material), sedimentary (rocks composed of products of mechanical weathering (sand, gravel, etc.) and chemical weathering (things precipitated from solution), and metamorphic (rocks produced by alteration of other rocks by heat and pressure.
3.1 Chemistry of Minerals
Rocks are composed of minerals that have a specific chemical composition. To understand mineral chemistry, it is essential to examine the fundamental unit of all matter, the atom.
3.1.1 The Atom

Video 3.1: Atomic orbitals.
If you are using an offline version of this text, access this YouTube video via the QR code.
Matter is made of atoms. Atoms consist of subatomic particles—protons, neutrons, and electrons. A simple model of the atom has a central nucleus composed of protons, which have positive charges, and neutrons which have no charge. A cloud of negatively charged electrons surrounds the nucleus, the number of electrons equaling the number of protons thus balancing the positive charge of the protons for a neutral atom. Protons and neutrons each have a mass number of 1. The mass of an electron is less than 1/1000th that of a proton or neutron, meaning most of the atom’s mass is in the nucleus.
3.1.2 Periodic Table of the Elements
Matter is composed of elements which are atoms that have a specific number of protons in the nucleus. This number of protons is called the Atomic Number for the element. For example, an oxygen atom has 8 protons and an iron atom has 26 protons. An element cannot be broken down chemically into a simpler form and retains unique chemical and physical properties. Each element behaves in a unique manner in nature. This uniqueness led scientists to develop a periodic table of the elements, a tabular arrangement of all known elements listed in order of their atomic number.
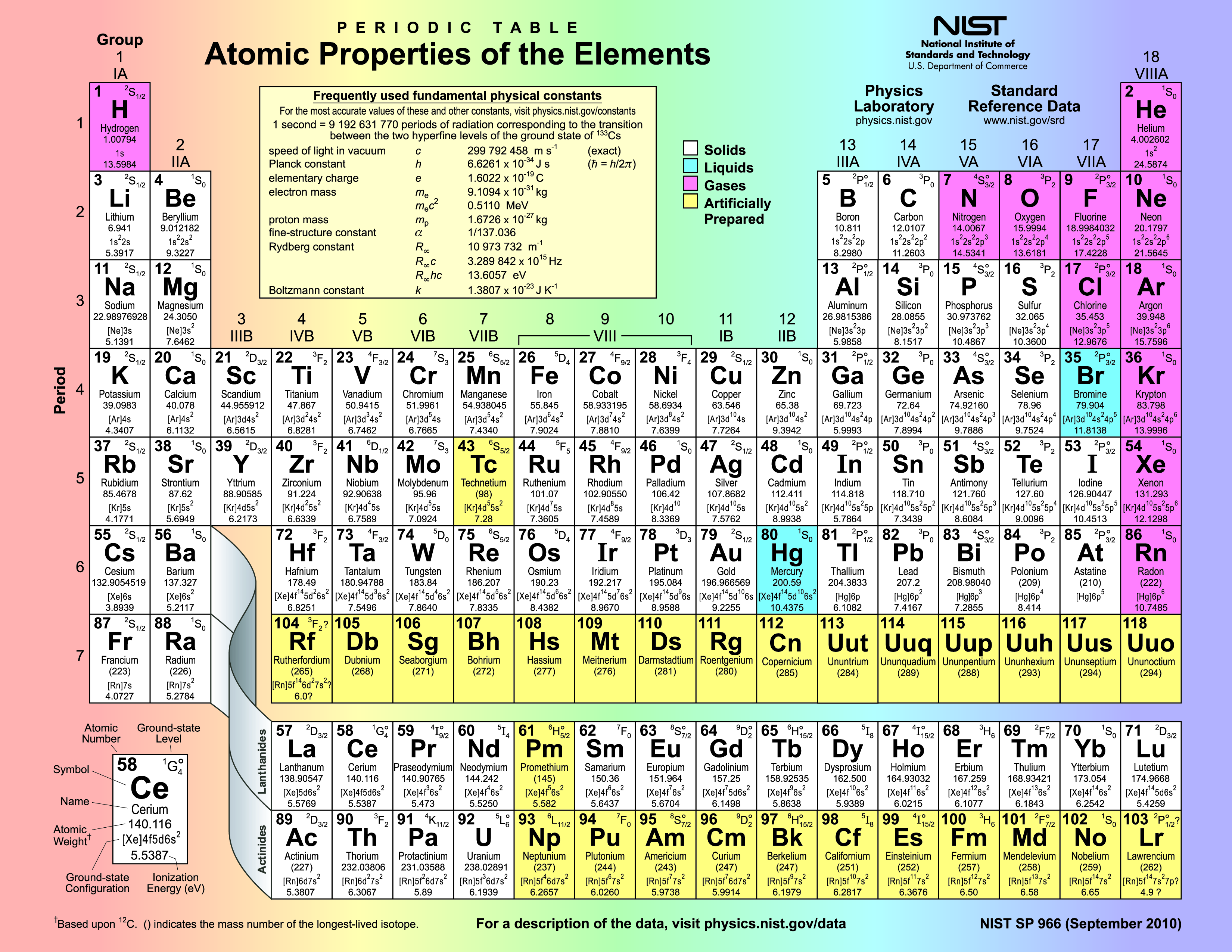
The first arrangement of elements into a periodic table was done by Dmitri Mendeleev in 1869 using the elements known at the time. In the periodic table, each element has a chemical symbol, name, atomic number, and atomic mass. The chemical symbol is an abbreviation for the element, often derived from a Latin or Greek name for the substance. The atomic number is the number of protons in the nucleus. The atomic mass is the number of protons and neutrons in the nucleus, each with a mass number of one. Since the mass of electrons is so much less than the protons and neutrons, the atomic mass is effectively the number of protons plus neutrons.
The atomic mass of natural elements represents an average mass of the atoms comprising that substance in nature and is usually not a whole number as seen on the periodic table, meaning that an element exists in nature with atoms having different numbers of neutrons. The differing number of neutrons affects the mass of an element in nature and the atomic mass number represents this average. This gives rise to the concept of isotope. Isotopes are forms of an element with the same number of protons but different numbers of neutrons. There are usually several isotopes for a particular element. For example, 98.9% of carbon atoms have 6 protons and 6 neutrons. This isotope of carbon is called carbon-12 (12C). A few carbon atoms, carbon-13 (13C), have 6 protons and 7 neutrons. A trace amount of carbon atoms, carbon-14 (14C), has 6 protons and 8 neutrons.
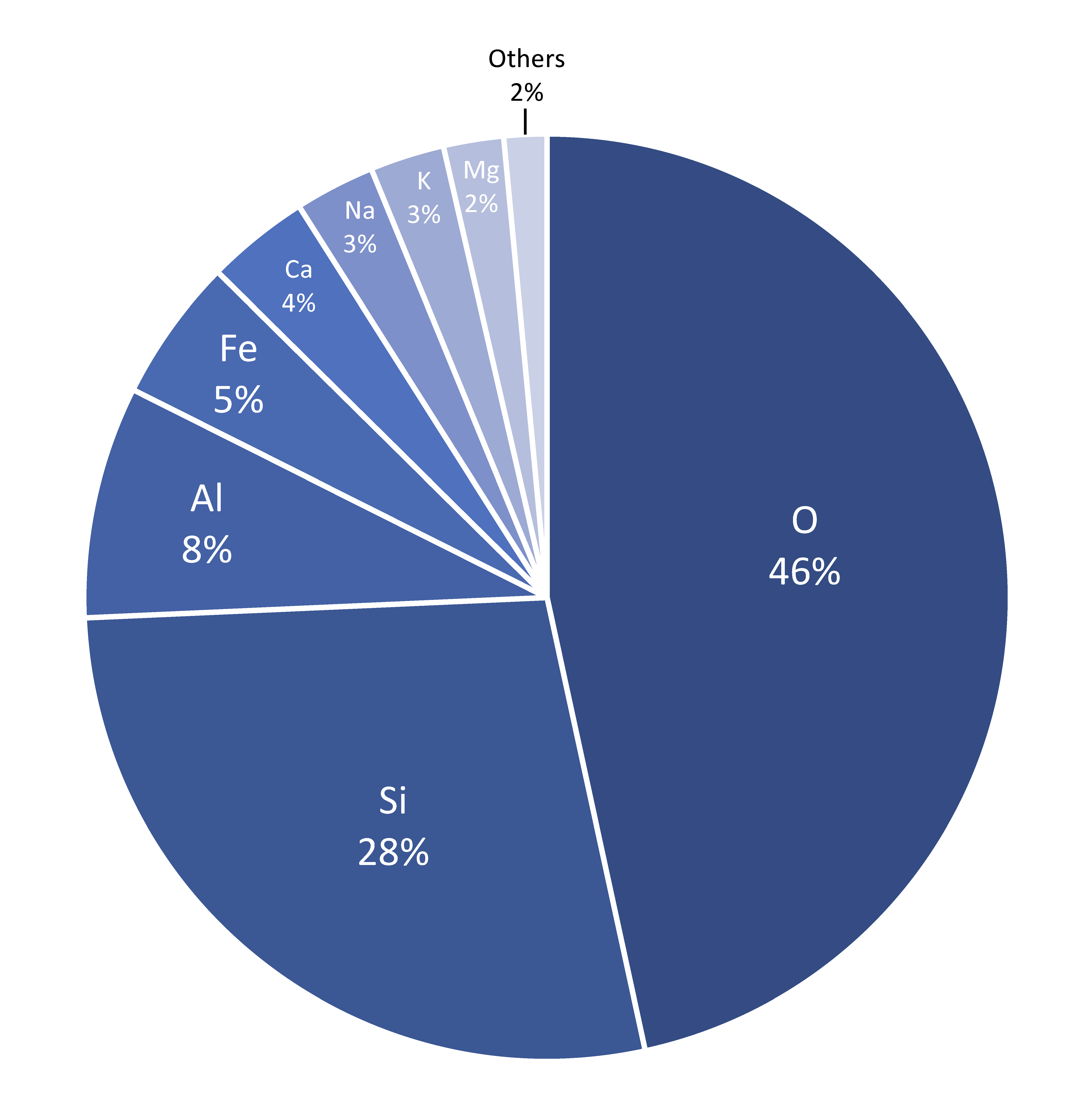
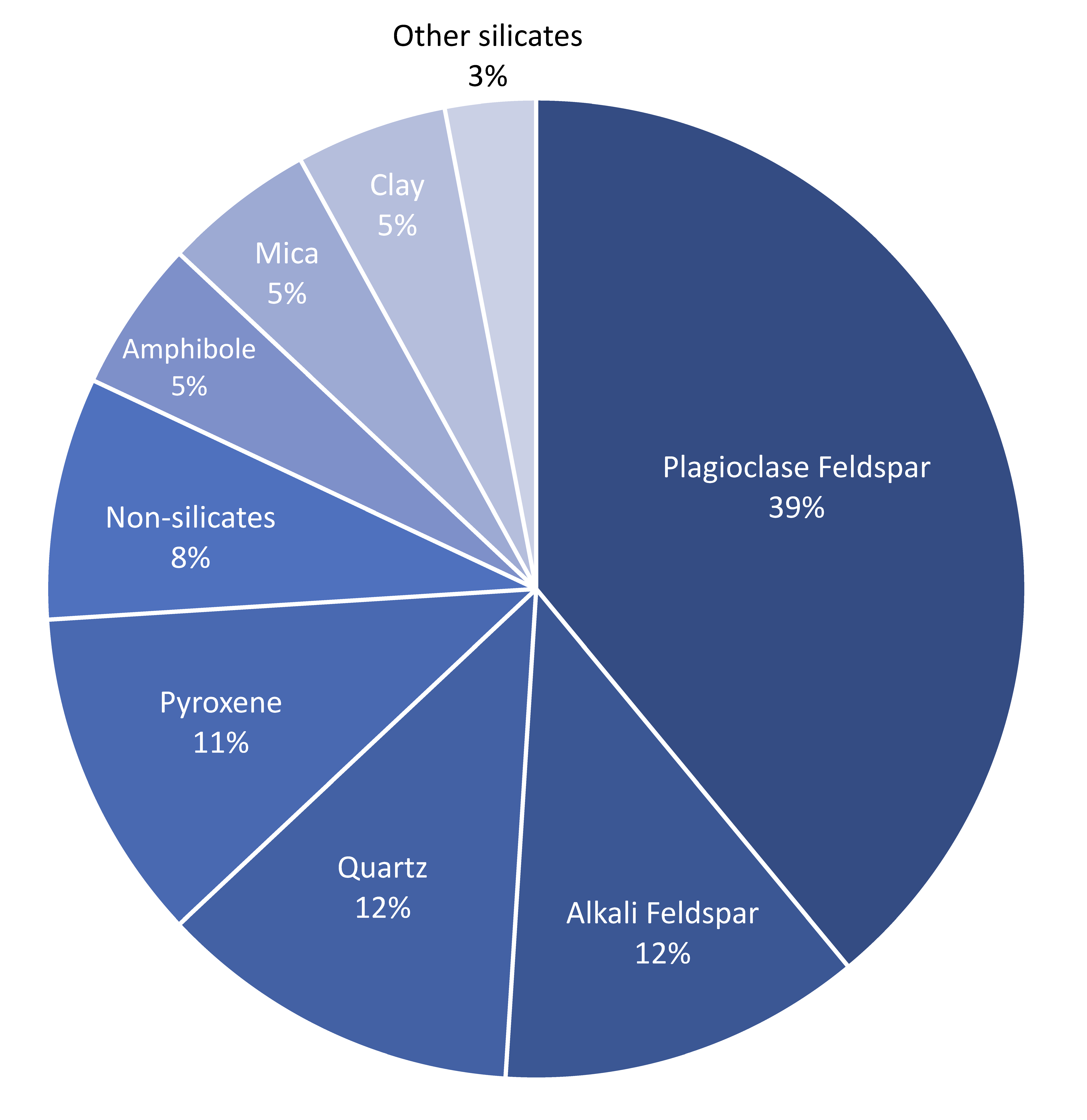
Among the 118 known elements, the heaviest are fleeting human creations known only in high energy particle accelerators, and they decay rapidly. The heaviest naturally occurring element is uranium, atomic number 92. The eight most abundant elements in Earth’s continental crust are shown in Table 1. These elements are found in the most common rock forming minerals.
| Element | Symbol | Abundance % |
|---|---|---|
| Oxygen | O | 47% |
| Silicon | Si | 28% |
| Aluminum | Al | 8% |
| Iron | Fe | 5% |
| Calcium | Ca | 4% |
| Sodium | Na | 3% |
| Potassium | K | 3% |
| Magnesium | Mg | 2% |
Table 3.1: Eight most abundant elements in the Earth’s continental crust (% by weight). All other elements are less than 1%. (Source: USGS).
3.1.3 Chemical Bonding
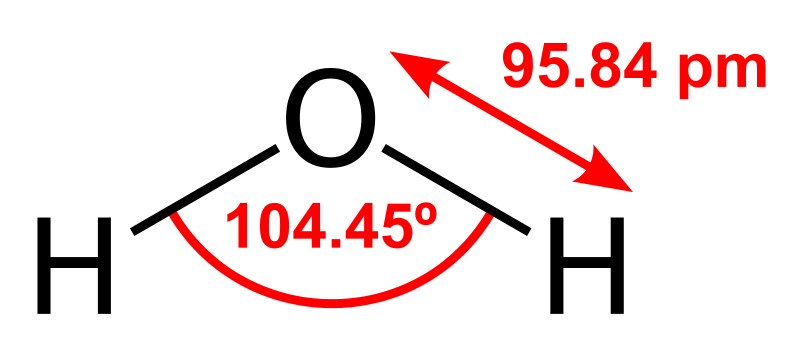
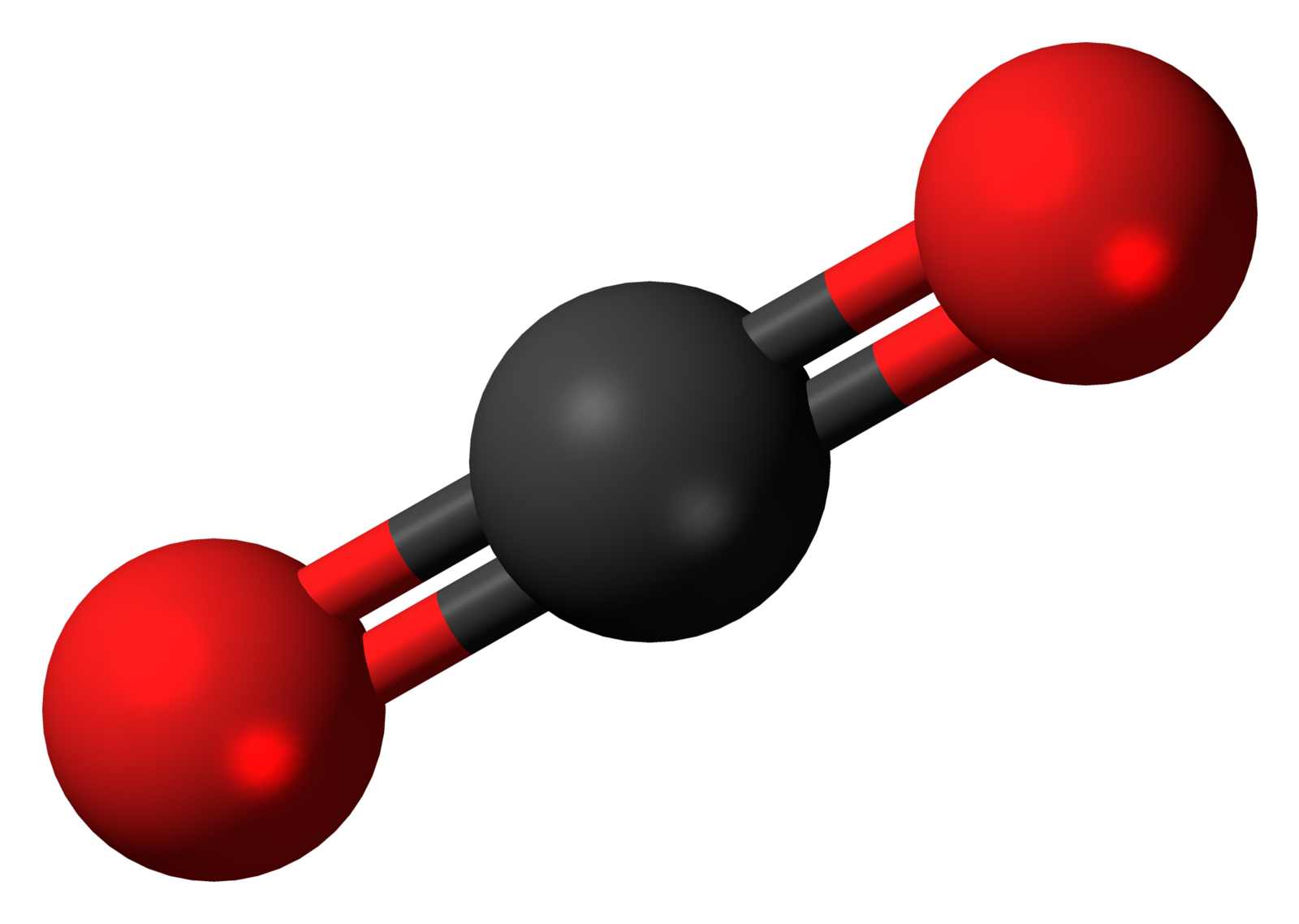
Most substances on Earth are compounds containing multiple elements. Chemical bonding describes how these atoms attach with each other to form compounds, such as sodium and chlorine combining to form NaCl, common table salt. Compounds that are held together by chemical bonds are called molecules. Water is a compound of hydrogen and oxygen in which two hydrogen atoms are covalently bonded with one oxygen making the water molecule. The oxygen we breathe is formed when one oxygen atom covalently bonds with another oxygen atom to make the molecule O2. The subscript 2 in the chemical formula indicates the molecule contains two atoms of oxygen.
Most minerals are also compounds of more than one element. The common mineral calcite has the chemical formula CaCO3 indicating the molecule consists of one calcium, one carbon, and three oxygen atoms. In calcite, one carbon and three oxygen atoms are held together by covalent bonds to form a molecular ion, called carbonate, which has a negative charge. Calcium as an ion has a positive charge of plus two. The two oppositely charged ions attract each other and combine to form the mineral calcite, CaCO3. The name of the chemical compound is calcium carbonate, where calcium is Ca and carbonate refers to the molecular ion CO3-2.
The mineral olivine has the chemical formula (Mg,Fe)2SiO4, in which one silicon and four oxygen atoms are bonded with two atoms of either magnesium or iron. The comma between iron (Fe) and magnesium (Mg) indicates the two elements can occupy the same location in the crystal structure and substitute for one another.
Valence and Charge
The electrons around the atom’s nucleus are located in shells representing different energy levels. The outermost shell is called the valence shell. Electrons in the valence shell are involved in chemical bonding. In 1913, Niels Bohr proposed a simple model of the atom that states atoms are more stable when their outermost shell is full. Atoms of most elements thus tend to gain or lose electrons so the outermost or valence shell is full. In Bohr’s model, the innermost shell can have a maximum of two electrons and the second and third shells can have a maximum of eight electrons. When the innermost shell is the valence shell, as in the case of hydrogen and helium, it obeys the octet rule when it is full with two electrons. For elements in higher rows, the octet rule of eight electrons in the valence shell applies.
The rows in the periodic table present the elements in order of atomic number and the columns organize elements with similar characteristics, such as the same number of electrons in their valence shells. Columns are often labeled from left to right with Roman numerals I to VIII, and Arabic numerals 1 through 18. The elements in columns I and II have 1 and 2 electrons in their respective valence shells and the elements in columns VI and VII have 6 and 7 electrons in their respective valence shells.
In row 3 and column I, sodium (Na) has 11 protons in the nucleus and 11 electrons in three shells—2 electrons in the inner shell, 8 electrons in the second shell, and 1 electron in the valence shell. To maintain a full outer shell of 8 electrons per the octet rule, sodium readily gives up that 1 electron so there are 10 total electrons. With 11 positively charged protons in the nucleus and 10 negatively charged electrons in two shells, sodium when forming chemical bonds is an ion with an overall net charge of +1.
All elements in column I have a single electron in their valence shell and a valence of 1. These other column I elements also readily give up this single valence electron and thus become ions with a +1 charge. Elements in column II readily give up 2 electrons and end up as ions with a charge of +2. Note that elements in columns I and II which readily give up their valence electrons, often form bonds with elements in columns VI and VII which readily take up these electrons. Elements in columns 3 through 15 are usually involved in covalent bonding. The last column 18 (VIII) contains the noble gases. These elements are chemically inert because the valence shell is already full with 8 electrons, so they do not gain or lose electrons. An example is the noble gas helium which has 2 valence electrons in the first shell. Its valence shell is therefore full. All elements in column VIII possess full valence shells and do not form bonds with other elements.
As seen above, an atom with a net positive or negative charge as a result of gaining or losing electrons is called an ion. In general the elements on the left side of the table lose electrons and become positive ions, called cations because they are attracted to the cathode in an electrical device. The elements on the right side tend to gain electrons. These are called anions because they are attracted to the anode in an electrical device. The elements in the center of the periodic table, columns 3 through 15, do not consistently follow the octet rule. These are called transition elements. A common example is iron, which has a +2 or +3 charge depending on the oxidation state of the element. Oxidized Fe+3 carries a +3 charge and reduced Fe+2 is +2. These two different oxidation states of iron often impart dramatic colors to rocks containing their minerals—the oxidized form producing red colors and the reduced form producing green.
Ionic Bonding
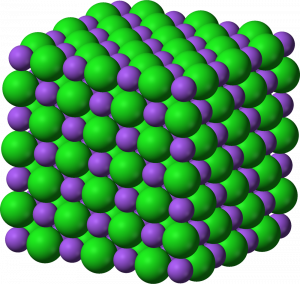
Ionic bonds, also called electron-transfer bonds, are formed by the electrostatic attraction between atoms having opposite charges. Atoms of two opposite charges attract each other electrostatically and form an ionic bond in which the positive ion transfers its electron (or electrons) to the negative ion which takes them up. Through this transfer both atoms thus achieve a full valence shell. For example one atom of sodium (Na+1) and one atom of chlorine (Cl-1) form an ionic bond to make the compound sodium chloride (NaCl). This is also known as the mineral halite or common table salt. Another example is calcium (Ca+2) and chlorine (Cl-1) combining to make the compound calcium chloride (CaCl2). The subscript 2 indicates two atoms of chlorine are ionically bonded to one atom of calcium.
Covalent Bonding
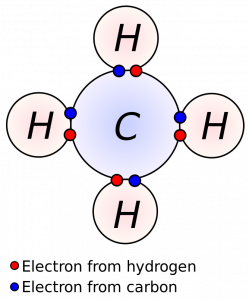
Ionic bonds are usually formed between a metal and a nonmetal. Another type, called a covalent or electron-sharing bond, commonly occurs between nonmetals. Covalent bonds share electrons between ions to complete their valence shells. For example, oxygen (atomic number 8) has 8 electrons—2 in the inner shell and 6 in the valence shell. Gases like oxygen often form diatomic molecules by sharing valence electrons. In the case of oxygen, two atoms attach to each other and share 2 electrons to fill their valence shells to become the common oxygen molecule we breathe (O2). Methane (CH4) is another covalently bonded gas. The carbon atom needs 4 electrons and each hydrogen needs 1. Each hydrogen shares its electron with the carbon to form a molecule as shown in the figure.

Take this quiz to check your comprehension of this section.
If you are using an offline version of this text, access the quiz for section 3.1 via the QR code.
3.2 Formation of Minerals
Minerals form when atoms bond together in a crystalline arrangement. Three main ways this occurs in nature are: 1) precipitation directly from an aqueous (water) solution with a temperature change, 2) crystallization from a magma with a temperature change, and 3) biological precipitation by the action of organisms.
3.2.1 Precipitation From Aqueous Solution

Solutions consist of ions or molecules, known as solutes, dissolved in a medium or solvent. In nature this solvent is usually water. Many minerals can be dissolved in water, such as halite or table salt, which has the composition sodium chloride, NaCl. The Na+1 and Cl-1 ions separate and disperse into the solution.
Precipitation is the reverse process, in which ions in solution come together to form solid minerals. Precipitation is dependent on the concentration of ions in solution and other factors such as temperature and pressure. The point at which a solvent cannot hold any more solute is called saturation. Precipitation can occur when the temperature of the solution falls, when the solute evaporates, or with changing chemical conditions in the solution. An example of precipitation in our homes is when water evaporates and leaves behind a rind of minerals on faucets, shower heads, and drinking glasses.
In nature, changes in environmental conditions may cause the minerals dissolved in water to form bonds and grow into crystals or cement grains of sediment together. In Utah, deposits of tufa formed from mineral-rich springs that emerged into the ice age Lake Bonneville. Now exposed in dry valleys, this porous tufa was a natural insulation used by pioneers to build their homes with a natural protection against summer heat and winter cold. The travertine terraces at Mammoth Hot Springs in Yellowstone Park are another example formed by calcite precipitation at the edges of the shallow spring-fed ponds.

Another example of precipitation occurs in the Great Salt Lake, Utah, where the concentration of sodium chloride and other salts is nearly eight times greater than in the world’s oceans. Streams carry salt ions into the lake from the surrounding mountains. With no other outlet, the water in the lake evaporates and the concentration of salt increases until saturation is reached and the minerals precipitate out as sediments. Similar salt deposits include halite and other precipitates, and occur in other lakes like Mono Lake in California and the Dead Sea.
3.2.2 Crystallization From Magma

Heat is energy that causes atoms in substances to vibrate. Temperature is a measure of the intensity of the vibration. If the vibrations are violent enough, chemical bonds are broken and the crystals melt releasing the ions into the melt. Magma is molten rock with freely moving ions. When magma is emplaced at depth or extruded onto the surface (then called lava), it starts to cool and mineral crystals can form.
3.2.3 Precipitation by Organisms

Many organisms build bones, shells, and body coverings by extracting ions from water and precipitating minerals biologically. The most common mineral precipitated by organisms is calcite, or calcium carbonate (CaCO3). Calcite is often precipitated by organisms as a polymorph called aragonite. Polymorphs are crystals with the same chemical formula but different crystal structures. Marine invertebrates such as corals and clams precipitate aragonite or calcite for their shells and structures. Upon death, their hard parts accumulate on the ocean floor as sediments, and eventually may become the sedimentary rock limestone. Though limestone can form inorganically, the vast majority is formed by this biological process. Another example is marine organisms called radiolaria, which are zooplankton that precipitate silica for their microscopic external shells. When the organisms die, the shells accumulate on the ocean floor and can form the sedimentary rock chert. An example of biologic precipitation from the vertebrate world is bone, which is composed mostly of a type of apatite, a mineral in the phosphate group. The apatite found in bones contains calcium and water in its structure and is called hydroxycarbonate apatite, Ca5(PO4)3(OH). As mentioned above, such substances are not technically minerals until the organism dies and these hard parts become fossils.

Take this quiz to check your comprehension of this section.
If you are using an offline version of this text, access the quiz for section 3.2 via the QR code.
3.3 Silicate Minerals
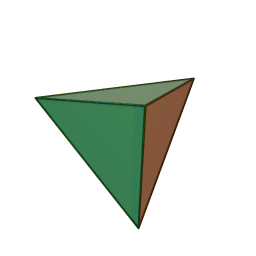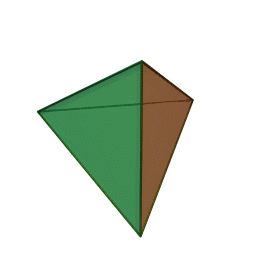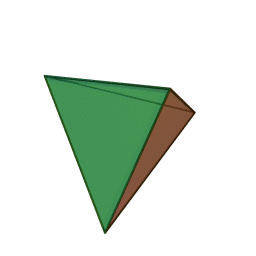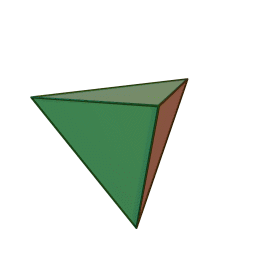
Minerals are categorized based on their composition and structure. Silicate minerals are built around a molecular ion called the silicon-oxygen tetrahedron. A tetrahedron has a pyramid-like shape with four sides and four corners. Silicate minerals form the largest group of minerals on Earth, comprising the vast majority of the Earth’s mantle and crust. Of the nearly four thousand known minerals on Earth, most are rare. There are only a few that make up most of the rocks likely to be encountered by surface dwelling creatures like us. These are generally called the rock-forming minerals.
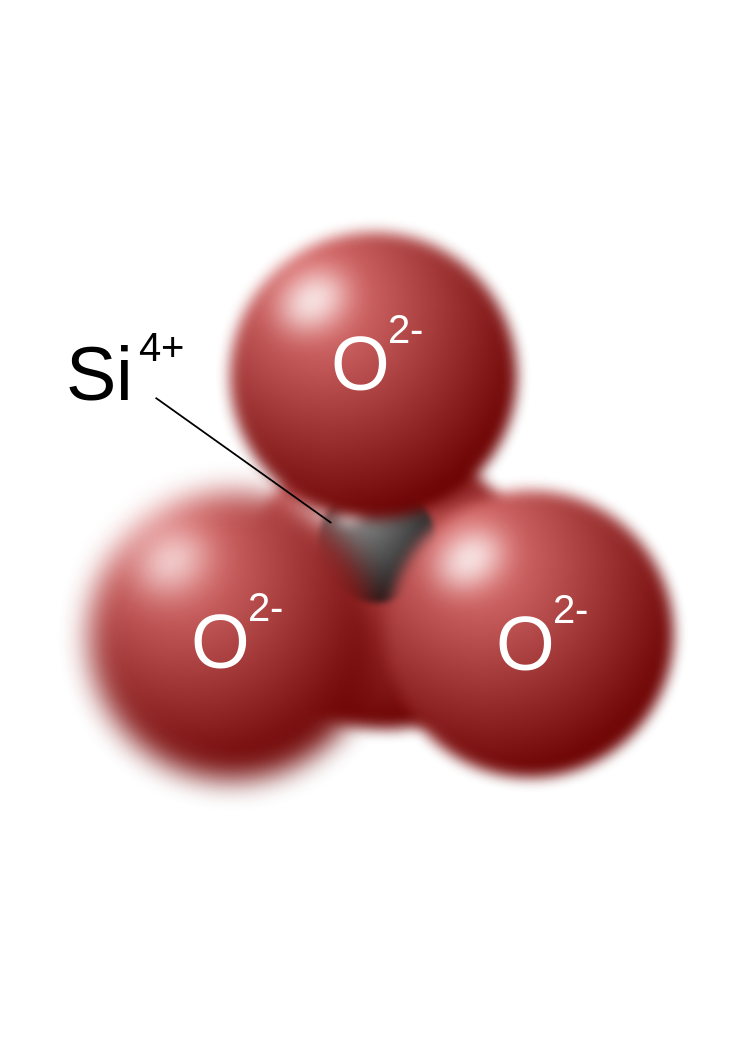
The silicon-oxygen tetrahedron (SiO4) consists of a single silicon atom at the center and four oxygen atoms located at the four corners of the tetrahedron. Each oxygen ion has a -2 charge and the silicon ion has a +4 charge. The silicon ion shares one of its four valence electrons with each of the four oxygen ions in a covalent bond to create a symmetrical geometric four-sided pyramid figure. Only half of the oxygen’s valence electrons are shared, giving the silicon-oxygen tetrahedron an ionic charge of -4. This silicon-oxygen tetrahedron forms bonds with many other combinations of ions to form the large group of silicate minerals.
The silicon ion is much smaller than the oxygen ions (see the figures) and fits into a small space in the center of the four large oxygen ions, seen if the top ball is removed (as shown in the figure to the right). Because only one of the valence electrons of the corner oxygens is shared, the silicon-oxygen tetrahedron has chemically active corners available to form bonds with other silica tetrahedra or other positively charged ions such as Al+3, Fe+2,+3, Mg+2, K+1, Na+1, and Ca+2. Depending on many factors, such as the original magma chemistry, silica-oxygen tetrahedra can combine with other tetrahedra in several different configurations. For example, tetrahedra can be isolated, attached in chains, sheets, or three dimensional structures. These combinations and others create the chemical structure in which positively charged ions can be inserted for unique chemical compositions forming silicate mineral groups.
3.3.1 The Dark Ferromagnesian Silicates

Olivine Family
Olivine is the primary mineral component in mantle rock such as peridotite and basalt. It is characteristically green when not weathered. The chemical formula is (Fe,Mg)2SiO4. As previously described, the comma between iron (Fe) and magnesium (Mg) indicates these two elements occur in a solid solution. Not to be confused with a liquid solution, a solid solution occurs when two or more elements have similar properties and can freely substitute for each other in the same location in the crystal structure.
Olivine is referred to as a mineral family because of the ability of iron and magnesium to substitute for each other. Iron and magnesium in the olivine family indicates a solid solution forming a compositional series within the mineral group which can form crystals of all iron as one end member and all mixtures of iron and magnesium in between to all magnesium at the other end member. Different mineral names are applied to compositions between these end members. In the olivine series of minerals, the iron and magnesium ions in the solid solution are about the same size and charge, so either atom can fit into the same location in the growing crystals. Within the cooling magma, the mineral crystals continue to grow until they solidify into igneous rock. The relative amounts of iron and magnesium in the parent magma determine which minerals in the series form. Other rarer elements with similar properties to iron or magnesium, like manganese (Mn), can substitute into the olivine crystalline structure in small amounts. Such ionic substitutions in mineral crystals give rise to the great variety of minerals and are often responsible for differences in color and other properties within a group or family of minerals. Olivine has a pure iron end-member (called fayalite) and a pure magnesium end-member (called forsterite). Chemically, olivine is mostly silica, iron, and magnesium and therefore is grouped among the dark-colored ferromagnesian (iron=ferro, magnesium=magnesian) or mafic minerals, a contraction of their chemical symbols Ma and Fe. Mafic minerals are also referred to as dark-colored ferromagnesian minerals. Ferro means iron and magnesian refers to magnesium. Ferromagnesian silicates tend to be more dense than non-ferromagnesian silicates. This difference in density ends up being important in controlling the behavior of the igneous rocks that are built from these minerals: whether a tectonic plate subducts or not is largely governed by the density of its rocks, which are in turn controlled by the density of the minerals that comprise them.
The crystal structure of olivine is built from independent silica tetrahedra. Minerals with independent tetrahedral structures are called neosilicates (or orthosilicates). In addition to olivine, other common neosilicate minerals include garnet, topaz, kyanite, and zircon.
Two other similar arrangements of tetrahedra are close in structure to the neosilicates and grade toward the next group of minerals, the pyroxenes. In a variation on independent tetrahedra called sorosilicates, there are minerals that share one oxygen between two tetrahedra, and include minerals like pistachio-green epidote, a gemstone. Another variation are the cyclosilicates, which as the name suggests, consist of tetrahedral rings, and include gemstones such as beryl, emerald, aquamarine, and tourmaline
Pyroxene Family

Pyroxene is another family of dark ferromagnesian minerals, typically black or dark green in color. Members of the pyroxene family have a complex chemical composition that includes iron, magnesium, aluminum, and other elements bonded to polymerized silica tetrahedra. Polymers are chains, sheets, or three-dimensional structures, and are formed by multiple tetrahedra covalently bonded via their corner oxygen atoms. Pyroxenes are commonly found in mafic igneous rocks such as peridotite, basalt, and gabbro, as well as metamorphic rocks like eclogite and blue schist.
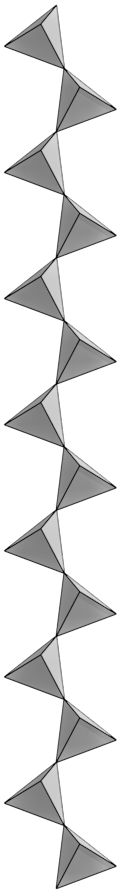
Pyroxenes are built from long, single chains of polymerized silica tetrahedra in which tetrahedra share two corner oxygens. The silica chains are bonded together into the crystal structures by metal cations. A common member of the pyroxene family is augite, itself containing several solid solution series with a complex chemical formula (Ca,Na)(Mg,Fe,Al,Ti)(Si,Al)2O6 that gives rise to a number of individual mineral names.
This single-chain crystalline structure bonds with many elements, which can also freely substitute for each other. The generalized chemical composition for pyroxene is XZ(Al,Si)2O6. X represents the ions Na, Ca, Mg, or Fe, and Z represents Mg, Fe, or Al. These ions have similar ionic sizes, which allows many possible substitutions among them. Although the cations may freely substitute for each other in the crystal, they carry different ionic charges that must be balanced out in the final crystalline structure. For example Na has a charge of +1, but Ca has charge of +2. If a Na+ ion substitutes for a Ca+2 ion, it creates an unequal charge that must be balanced by other ionic substitutions elsewhere in the crystal. Note that ionic size is more important than ionic charge for substitutions to occur in solid solution series in crystals.
Amphibole Family


Amphibole minerals are built from polymerized double silica chains and they are also referred to as inosilicates. Imagine two pyroxene chains that connect together by sharing a third oxygen on each tetrahedra. Amphiboles are usually found in igneous and metamorphic rocks and typically have a long-bladed crystal habit. The most common amphibole, hornblende, is usually black; however, they come in a variety of colors depending on their chemical composition. The metamorphic rock, amphibolite, is primarily composed of amphibole minerals.

Amphiboles are composed of iron, magnesium, aluminum, and other cations bonded with silica tetrahedra. These dark ferromagnesian minerals are commonly found in gabbro, baslt, diorite, and often form the black specks in granite. Their chemical formula is very complex and generally written as (RSi4O11)2, where R represents many different cations. For example, it can also be written more exactly as AX2Z5((Si,Al,Ti)8O22)(OH,F,Cl,O)2. In this formula A may be Ca, Na, K, Pb, or blank; X equals Li, Na, Mg, Fe, Mn, or Ca; and Z is Li, Na, Mg, Fe, Mn, Zn, Co, Ni, Al, Cr, Mn, V, Ti, or Zr. The substitutions create a wide variety of colors such as green, black, colorless, white, yellow, blue, or brown. Amphibole crystals can also include hydroxide ions (OH–), which occurs from an interaction between the growing minerals and water dissolved in magma.
3.3.2 Sheet Silicates


Sheet silicates are built from tetrahedra which share all three of their bottom corner oxygens thus forming sheets of tetrahedra with their top corners available for bonding with other atoms. Micas and clays are common types of sheet silicates, also known as phyllosilicates. Mica minerals are usually found in igneous and metamorphic rocks, while clay minerals are more often found in sedimentary rocks. Two frequently found micas are dark-colored biotite, frequently found in granite, and light-colored muscovite, found in the metamorphic rock called schist.
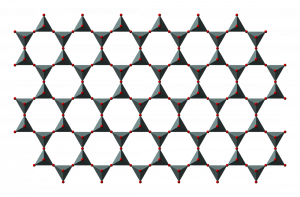
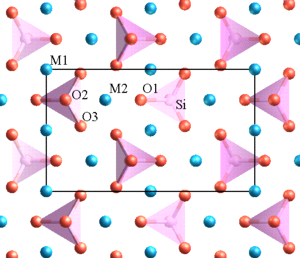
Chemically, sheet silicates usually contain silicon and oxygen in a 2:5 ratio (Si4O10). Micas contain mostly silica, aluminum, and potassium. Biotite mica has more iron and magnesium and is considered a ferromagnesian silicate mineral. Muscovite micas belong to the felsic silicate minerals. Felsic is a contraction formed from feldspar, the dominant mineral in felsic rocks.
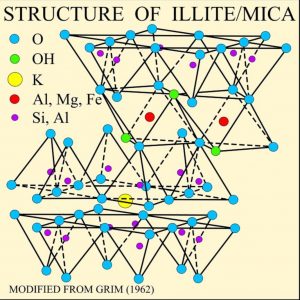
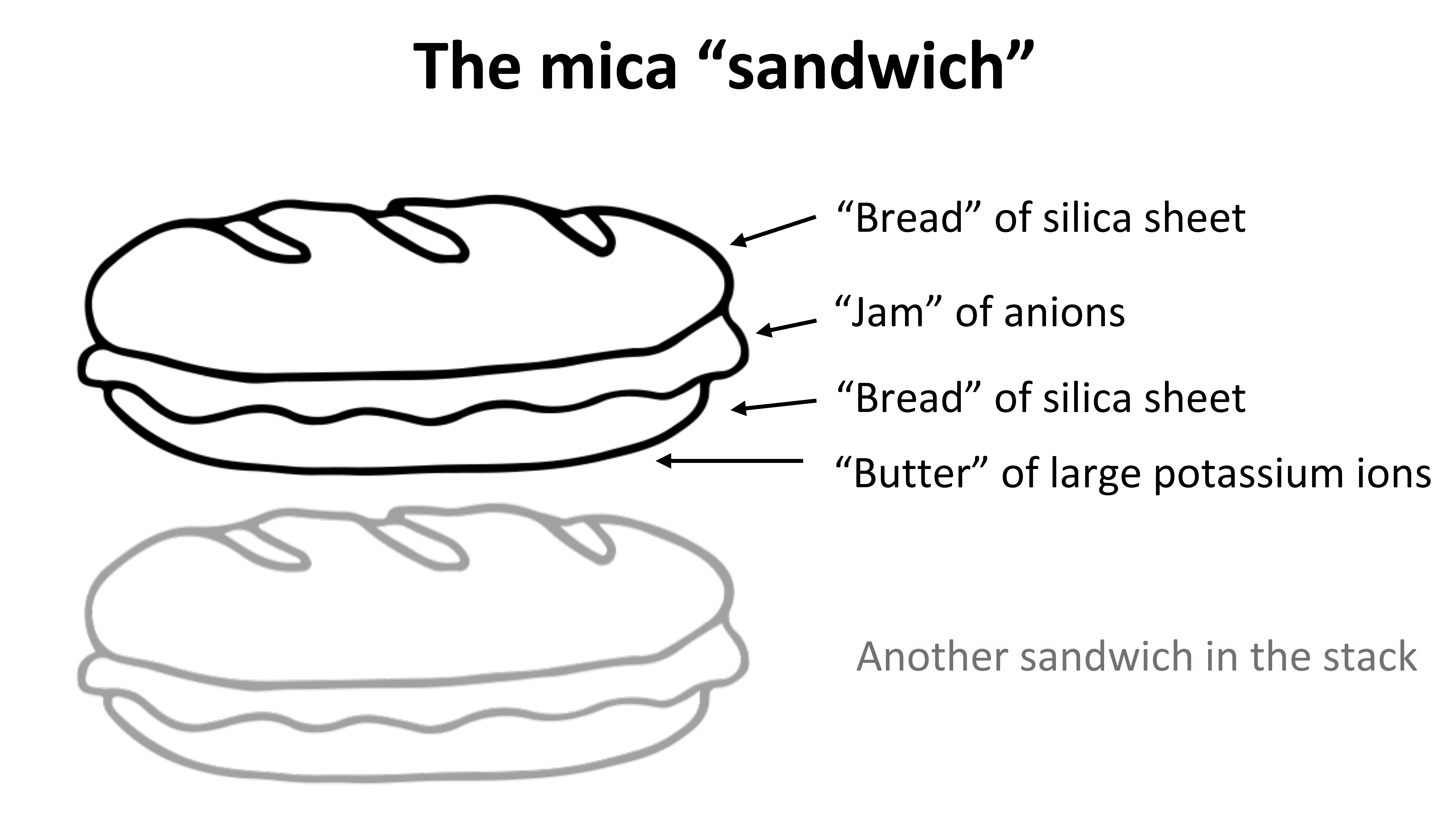
The illustration of the crystalline structure of mica shows the corner O atoms bonded with K, Al, Mg, Fe, and Si atoms, forming polymerized sheets of linked tetrahedra, with an octahedral layer of Fe, Mg, or Al, between them. The yellow potassium ions form Van der Waals bonds (attraction and repulsion between atoms, molecules, and surfaces) and hold the sheets together. Van der Waals bonds differ from covalent and ionic bonds, and exist here between the sandwiches, holding them together into a stack of sandwiches. The Van der Waals bonds are weak compared to the bonds within the sheets, allowing the sandwiches to be separated along the potassium layers. This gives mica its characteristic property of easily cleaving into sheets.
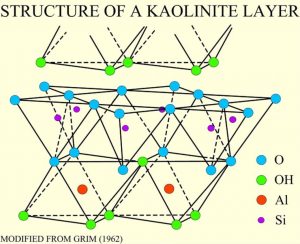
Clays minerals occur in sediments formed by the weathering of rocks and are another family of silicate minerals with a tetrahedral sheet structure. Clay minerals form a complex family, and are an important component of many sedimentary rocks. Other sheet silicates include serpentine and chlorite, found in metamorphic rocks.
Clay minerals are composed of hydrous aluminum silicates. One type of clay, kaolinite, has a structure like an open-faced sandwich, with the bread being a single layer of silicon-oxygen tetrahedra and a layer of aluminum as the spread in an octahedral configuration with the top oxygens of the sheets.
3.3.3 Framework Silicates

Quartz and feldspar are the two most abundant minerals in the continental crust. In fact, feldspar itself is the single most abundant mineral in the Earth’s crust. There are two types of feldspar, one containing potassium and abundant in felsic rocks of the continental crust, and the other with sodium and calcium abundant in the mafic rocks of oceanic crust. Together with quartz, these minerals are classified as framework silicates. They are built with a three-dimensional framework of silica tetrahedra in which all four corner oxygens are shared with adjacent tetrahedra. Within these frameworks in feldspar are holes and spaces into which other ions like aluminum, potassium, sodium, and calcium can fit giving rise to a variety of mineral compositions and mineral names.
Feldspars are usually found in igneous rocks, such as granite, rhyolite, and basalt as well as metamorphic rocks and detrital sedimentary rocks. Detrital sedimentary rocks are composed of mechanically weathered rock particles, like sand and gravel. Quartz is especially abundant in detrital sedimentary rocks because it is very resistant to disintegration by weathering. While quartz is the most abundant mineral on the Earth’s surface, due to its durability, the feldspar minerals are the most abundant minerals in the Earth’s crust, comprising roughly 50% of the total minerals that make up the crust.

Quartz is composed of pure silica, SiO2, with the tetrahedra arranged in a three dimensional framework. Impurities consisting of atoms within this framework give rise to many varieties of quartz among which are gemstones like amethyst, rose quartz, and citrine. Feldspars are mostly silica with aluminum, potassium, sodium, and calcium. Orthoclase feldspar (KAlSi3O8), also called potassium feldspar or K-spar, is made of silica, aluminum, and potassium. Quartz and orthoclase feldspar are felsic minerals. Felsic is the compositional term applied to continental igneous minerals and rocks that contain an abundance of silica. Another feldspar is plagioclase with the formula (Ca,Na)AlSi3O8, the solid solution (Ca,Na) indicating a series of minerals, one end of the series with calcium CaAl2Si2O8, called anorthite, and the other end with sodium NaAlSi3O8, called albite. Note how the mineral accommodates the substitution of Ca++ and Na+. Minerals in this solid solution series have different mineral names.
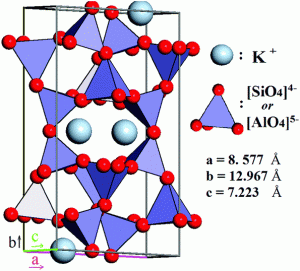
Note that aluminum, which has a similar ionic size to silicon, can substitute for silicon inside the tetrahedra. Because potassium ions are so much larger than sodium and calcium ions, which are very similar in size, the inability of the crystal lattice to accommodate both potassium and sodium/calcium gives rise to the two families of feldspar, orthoclase and plagioclase respectively. Framework silicates are called tectosilicates and include the alkali metal-rich feldspathoids and zeolites.

Take this quiz to check your comprehension of this section.
If you are using an offline version of this text, access the quiz for section 3.3 via the QR code.
3.4 Non-silicate Minerals

The crystal structure of non-silicate minerals (see table) does not contain silica-oxygen tetrahedra. Many non-silicate minerals are economically important and provide metallic resources such as copper, lead, and iron. They also include valuable non-metallic products such as salt, construction materials, and fertilizer.
| Mineral group | Examples | Formula | Uses |
|---|---|---|---|
| Native elements | Gold, silver, copper | Au, Ag, Cu | Jewelry, coins, industry |
| Carbonates | Calcite, dolomite | CaCO3, CaMg(CO3)2 | Lime, Portland cement |
| Oxides | Hematite, magnetite, bauxite | Fe2O3, Fe3O4, a mixture of aluminum oxides | Ores of iron & aluminum, pigments |
| Halides | Halite, sylvite | NaCl, KCl | Table salt, fertilizer |
| Sulfides | Galena, chalcopyrite, cinnabar | PbS, CuFeS2, HgS | Ores of lead, copper, mercury |
| Sulphates | Gypsum, epsom salts | CaSo4 · 2H2O, MgSO4 · 7H2O | Sheetrock, therapeutic soak |
| Phosphates | Apatite | Ca5(PO4)3(F,Cl,OH) | Fertilizer, teeth, bones |
Table 3.2: Common non-silicatemineral groups.
3.4.1 Carbonates


Calcite (CaCO3) and dolomite (CaMg(CO3)2) are the two most frequently occurring carbonate minerals, and usually occur in sedimentary rocks, such as limestone and dolostone rocks, respectively. Some carbonate rocks, such calcite and dolomite, are formed via evaporation and precipitation. However, most carbonate-rich rocks, such as limestone, are created by the lithification of fossilized marine organisms. These organisms, including those we can see and many microscopic organisms, have shells or exoskeletons consisting of calcium carbonate (CaCO3). When these organisms die, their remains accumulate on the floor of the water body in which they live and the soft body parts decompose and dissolve away. The calcium carbonate hard parts become included in the sediments, eventually becoming the sedimentary rock called limestone. While limestone may contain large, easy to see fossils, most limestones contain the remains of microscopic creatures and thus originate from biological processes.
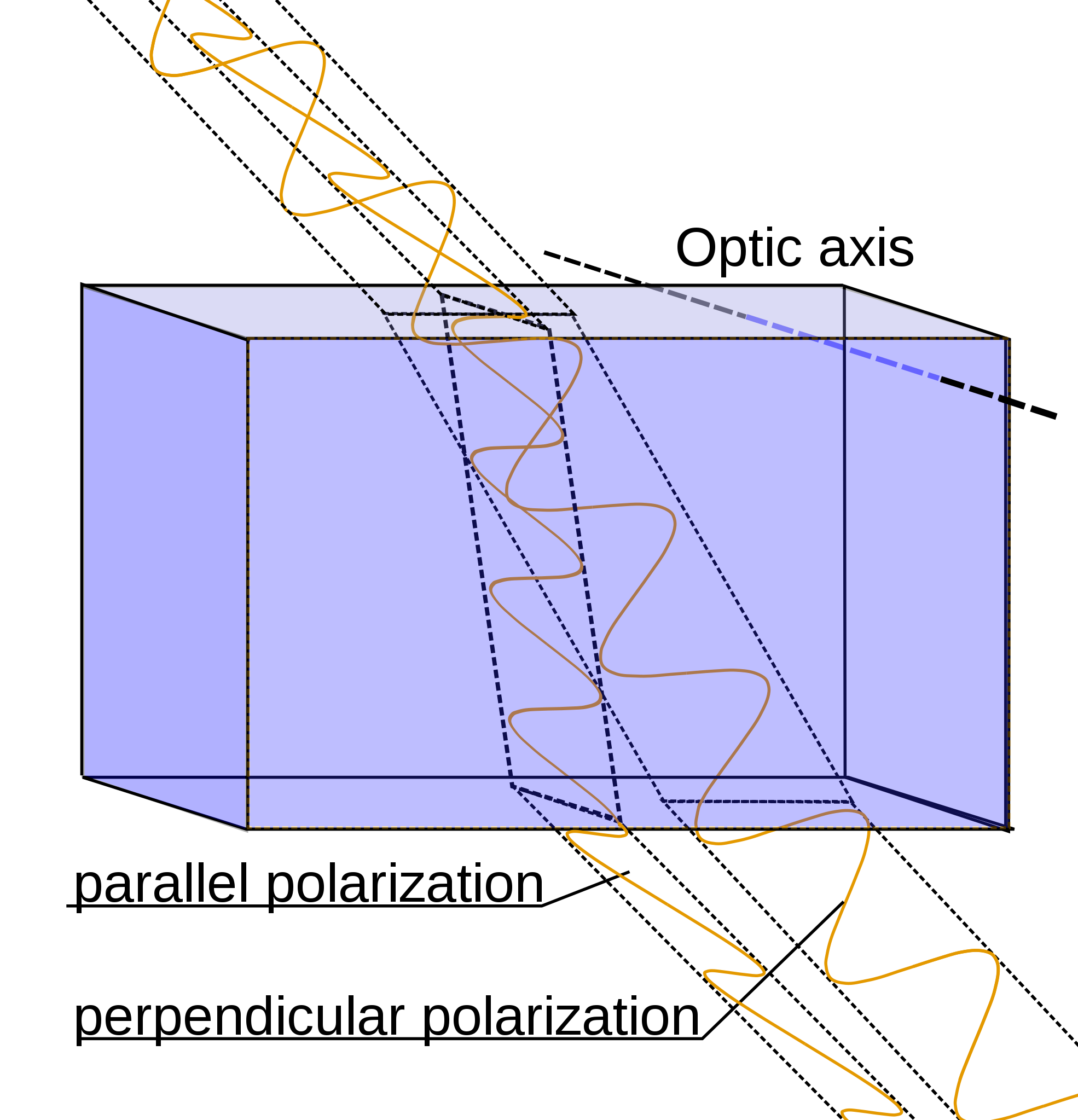
Calcite crystals show an interesting property called birefringence, meaning they polarize light into two wave components vibrating at right angles to each other. As the two light waves pass through the crystal, they travel at different velocities and are separated by refraction into two different travel paths. In other words, the crystal produces a double image of objects viewed through it. Because they polarize light, calcite crystals are used in special petrographic microscopes for studying minerals and rocks.
Many non-silicate minerals are referred to as salts. The term salts used here refers to compounds made by replacing the hydrogen in natural acids. The most abundant natural acid is carbonic acid that forms by the solution of carbon dioxide in water. Carbonate minerals are salts built around the carbonate ion (CO3-2) where calcium and/or magnesium replace the hydrogen in carbonic acid (H2CO3). Calcite and a closely related polymorph aragonite are secreted by organisms to form shells and physical structures like corals. Many such creatures draw both calcium and carbonate from dissolved bicarbonate ions (HCO3–) in ocean water. As seen in the mineral identification section below, calcite is easily dissolved in acid and thus effervesces in dilute hydrochloric acid (HCl). Small dropper bottles of dilute hydrochloric acid are often carried by geologists in the field as well as used in mineral identification labs.
Other salts include halite (NaCl) in which sodium replaces the hydrogen in hydrochloric acid and gypsum (Ca[SO4] • 2 H2O) in which calcium replaces the hydrogen in sulfuric acid. Note that some water molecules are also included in the gypsum crystal. Salts are often formed by evaporation and are called evaporite minerals.
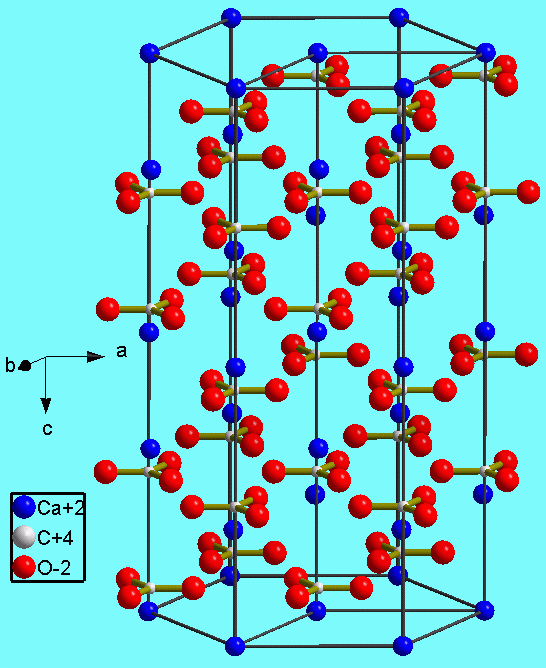
The figure shows the crystal structure of calcite (CaCO3). Like silicon, carbon has four valence electrons. The carbonate unit consists of carbon atoms (tiny white dots) covalently bonded to three oxygen atoms (red), one oxygen sharing two valence electrons with the carbon and the other two sharing one valence electron each with the carbon, thus creating triangular units with a charge of -2. The negatively charged carbonate unit forms an ionic bond with the Ca ion (blue), which as a charge of +2.
3.4.2 Oxides, Halides, and Sulfides

After carbonates, the next most common non-silicate minerals are the oxides, halides, and sulfides.
Oxides consist of metal ions covalently bonded with oxygen. The most familiar oxide is rust, which is a combination of iron oxides (Fe2O3) and hydrated oxides. Hydrated oxides form when iron is exposed to oxygen and water. Iron oxides are important for producing metallic iron. When iron oxide or ore is smelted, it produces carbon dioxide (CO2) and metallic iron.
The red color in rocks is usually due to the presence of iron oxides. For example, the red sandstone cliffs in Zion National Park and throughout Southern Utah consist of white or colorless grains of quartz coated with iron oxide which serve as cementing agents holding the grains together.

Other iron oxides include limonite, magnetite, and hematite. Hematite occurs in many different crystal forms. The massive form shows no external structure. Botryoidal hematite shows large concentric blobs. Specular hematite looks like a mass of shiny metallic crystals. Oolitic hematite looks like a mass of dull red fish eggs. These different forms of hematite are polymorphs and all have the same formula, Fe2O3.
Other common oxide minerals include:
- ice (H2O), an oxide of hydrogen
- bauxite (Al2H2O4), hydrated oxides of aluminum, an ore for producing metallic aluminum
- corundum (Al2O3), which includes ruby and sapphire gemstones.

The halides consist of halogens in column VII, usually fluorine or chlorine, ionically bonded with sodium or other cations. These include halite or sodium chloride (NaCl), common table salt; sylvite or potassium chloride (KCl); and fluorite or calcium fluoride (CaF2).

Halide minerals usually form from the evaporation of sea water or other isolated bodies of water. A well-known example of halide mineral deposits created by evaporation is the Bonneville Salt Flats, located west of the Great Salt Lake in Utah (see figure 3.39).

Many important metal ores are sulfides, in which metals are bonded to sulfur. Significant examples include: galena (lead sulfide), sphalerite (zinc sulfide), pyrite (iron sulfide, sometimes called “fool’s gold”), and chalcopyrite (iron-copper sulfide). Sulfides are well known for being important ore minerals. For example, galena is the main source of lead, sphalerite is the main source of zinc, and chalcopyrite is the main copper ore mineral mined in porphyry deposits like the Bingham mine (see chapter 16). The largest sources of nickel, antimony, molybdenum, arsenic, and mercury are also sulfides.
3.4.3 Sulfates

Sulfate minerals contain a metal ion, such as calcium, bonded to a sulfate ion. The sulfate ion is a combination of sulfur and oxygen (SO4–2). The sulfate mineral gypsum (CaSO4 • 2H2O) is used in construction materials such as plaster and drywall. Gypsum is often formed from evaporating water and usually contains water molecules in its crystalline structure. The 2H2O in the formula indicates the water molecules are whole H2O. This is different from minerals like amphibole, which contain a hydroxide ion (OH–) that is derived from water, but is missing a hydrogen ion (H+). The calcium sulfate without water is a different mineral than gypsum called anhydrite (CaSO4).
3.4.4 Phosphates

Phosphate minerals have a tetrahedral phosphate unit (PO4-3) combined with various anions and cations. In some cases arsenic or vanadium can substitute for phosphorus. Phosphates are an important ingredient of fertilizers as well as detergents, paint, and other products. The best known phosphate mineral is apatite, Ca5(PO4)3(F,Cl,OH), variations of which are found in teeth and bones. The gemstone turquoise [CuAl6(PO4)4(OH)8·4H2O ] is a copper-rich phosphate mineral that, like gypsum, contains water molecules.
3.4.5 Native Element Minerals


Native element minerals, usually metals, occur in nature in a pure or nearly pure state. Gold is an example of a native element mineral; it is not very reactive and rarely bonds with other elements so it is usually found in an isolated or pure state. The non-metallic and poorly-reactive mineral carbon is often found as a native element, such as graphite and diamonds. Mildly reactive metals like silver, copper, platinum, mercury, and sulfur sometimes occur as native element minerals. Reactive metals such as iron, lead, and aluminum almost always bond to other elements and are rarely found in a native state.

Take this quiz to check your comprehension of this section.
If you are using an offline version of this text, access the quiz for section 3.4 via the QR code.
3.5 Identifying Minerals

Geologists identify minerals by their physical properties. In the field, where geologists may have limited access to advanced technology and powerful machines, they can still identify minerals by testing several physical properties: luster and color, streak, hardness, crystal habit, cleavage and fracture, and some special properties. Only a few common minerals make up the majority of Earth’s rocks and are usually seen as small grains in rocks. Of the several properties used for identifying minerals, it is good to consider which will be most useful for identifying them in small grains surrounded by other minerals.
3.5.1 Luster and Color

The first thing to notice about a mineral is its surface appearance, specifically luster and color. Luster describes how the mineral looks. Metallic luster looks like a shiny metal such as chrome, steel, silver, or gold. Submetallic luster has a duller appearance. Pewter, for example, shows submetallic luster.

Nonmetallic luster doesn’t look like a metal and may be described as vitreous (glassy), earthy, silky, pearly, and other surface qualities. Nonmetallic minerals may be shiny, although their vitreous shine is different from metallic luster. See the table for descriptions and examples of nonmetallic luster.
| Luster | Description | Image | Image description |
|---|---|---|---|
| Vitreous/glassy | Surface is shiny like glass |  |
Quartz crystals showing vitreous luster |
| Earthy/dull | Dull, like dried mud or clay |  |
Kaolin specimen showing dull or earthy luster |
| Silky | Soft shine like silk fabric |  |
Gypsum specimen showing silky luster |
| Pearly | Like the inside of a clam shell or mother-of-pearl |  |
Mica specimen showing pearly luster |
| Submetallic | Has the appearance of dull metal, like pewter. These minerals would usually still be considered metallic. Submetallic appearance can occur in metallic minerals because of weathering. |  |
Sphalerite specimen showing submetallic luster |
Table 3.3: Nonmetallic luster descriptions and examples.

Surface color may be helpful in identifying minerals, although it can be quite variable within the same mineral family. Mineral colors are affected by the main elements as well as impurities in the crystals. These impurities may be rare elements—like manganese, titanium, chromium, or lithium—even other molecules that are not normally part of the mineral formula. For example, the incorporation of water molecules gives quartz, which is normally clear, a milky color.
Some minerals predominantly show a single color. Malachite and azurite are green and blue, respectively, because of their copper content. Other minerals have a predictable range of colors due to elemental substitutions, usually via a solid solution. Feldspars, the most abundant minerals in the earth’s crust, are complex, have solid solution series, and present several colors including pink, white, green, gray and others. Other minerals also come in several colors, influenced by trace amounts of several elements. The same element may show up as different colors, in different minerals. With notable exceptions, color is usually not a definitive property of minerals. For identifying many minerals. a more reliable indicator is streak, which is the color of the powdered mineral.
3.5.2 Streak

Streak examines the color of a powdered mineral, and can be seen when a mineral sample is scratched or scraped on an unglazed porcelain streak plate. A paper page in a field notebook may also be used for the streak of some minerals. Minerals that are harder than the streak plate will not show streak, but will scratch the porcelain. For these minerals, a streak test can be obtained by powdering the mineral with a hammer and smearing the powder across a streak plate or notebook paper.
While mineral surface colors and appearances may vary, their streak colors can be diagnostically useful. An example of this property is seen in the iron-oxide mineral hematite. Hematite occurs in a variety of forms, colors and lusters, from shiny metallic silver to earthy red-brown, and different physical appearances. A hematite streak is consistently reddish brown, no matter what the original specimen looks like. Iron sulfide or pyrite, is a brassy metallic yellow. Commonly named fool’s gold, pyrite has a characteristic black to greenish-black streak.
3.5.3 Hardness
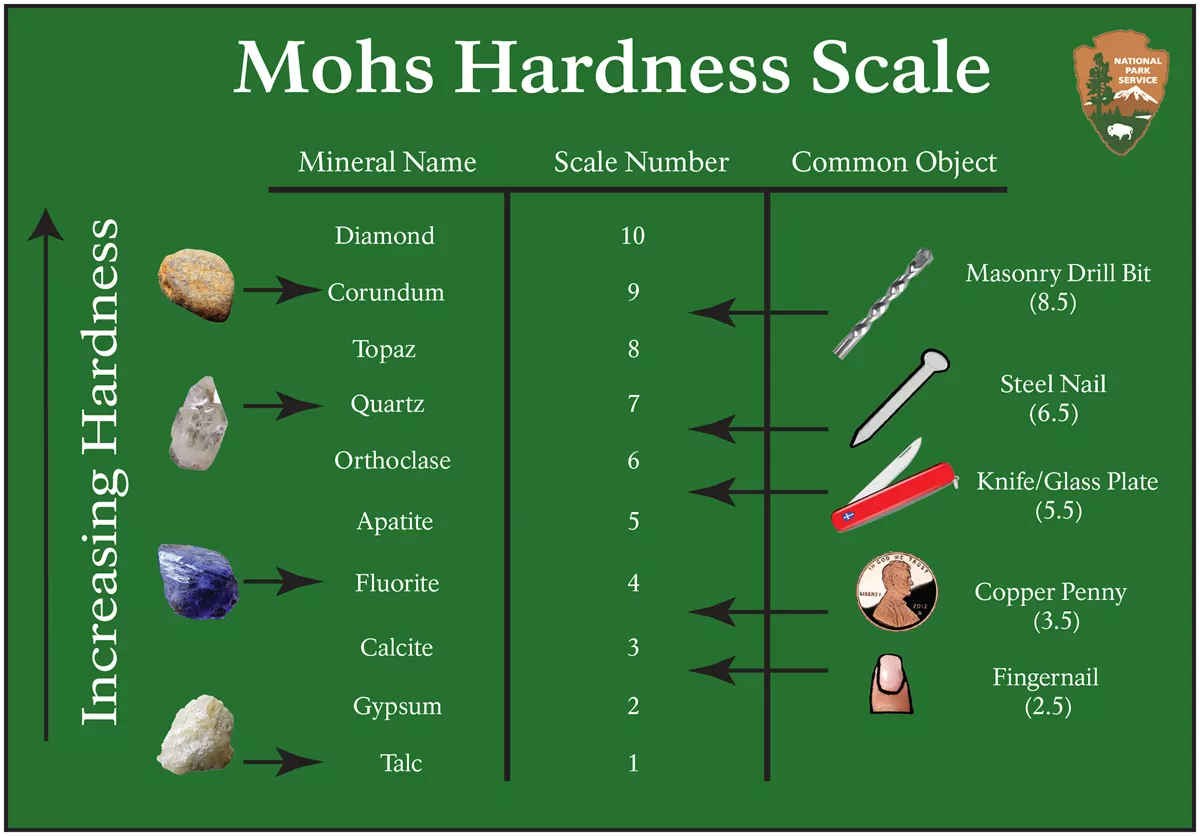
Hardness measures the ability of a mineral to scratch other substances. The Mohs Hardness Scale gives a number showing the relative scratch-resistance of minerals when compared to a standardized set of minerals of increasing hardness. The Mohs scale was developed by German geologist Fredrick Mohs in the early 20th century, although the idea of identifying minerals by hardness goes back thousands of years. Mohs hardness values are determined by the strength of a mineral’s atomic bonds.
The figure shows the minerals associated with specific hardness values, together with some common items readily available for use in field testing and mineral identification. The hardness values run from 1 to 10, with 10 being the hardest; however, the scale is not linear. Diamond defines a hardness of 10 and is actually about four times harder than corundum, which is 9. A steel pocketknife blade, which has a hardness value of 5.5, separates between hard and soft minerals on many mineral identification keys.
3.5.4 Crystal Habit
Minerals can be identified by crystal habit, how their crystals grow and appear in rocks. Crystal shapes are determined by the arrangement of the atoms within the crystal structure. For example, a cubic arrangement of atoms gives rise to a cubic-shaped mineral crystal. Crystal habit refers to typically observed shapes and characteristics; however, they can be affected by other minerals crystallizing in the same rock. When minerals are constrained so they do not develop their typical crystal habit, they are called anhedral. Subhedral crystals are partially formed shapes. For some minerals characteristic crystal habit is to grow crystal faces even when surrounded by other crystals in rock. An example is garnet. Minerals grown freely where the crystals are unconstrained and can take characteristic shapes often form crystal faces. A euhedral crystal has a perfectly formed, unconstrained shape. Some minerals crystallize in such tiny crystals, they do not show a specific crystal habit to the naked eye. Other minerals, like pyrite, can have an array of different crystal habits, including cubic, dodecahedral, octahedral, and massive. The table lists typical crystal habits of various minerals.
| Habit | Image | Examples |
|---|---|---|
| Bladed Long and flat crystals |
 |
Kyanite, amphibole, gypsum |
| Botryoidal/mammillary Blobby, circular crystals |
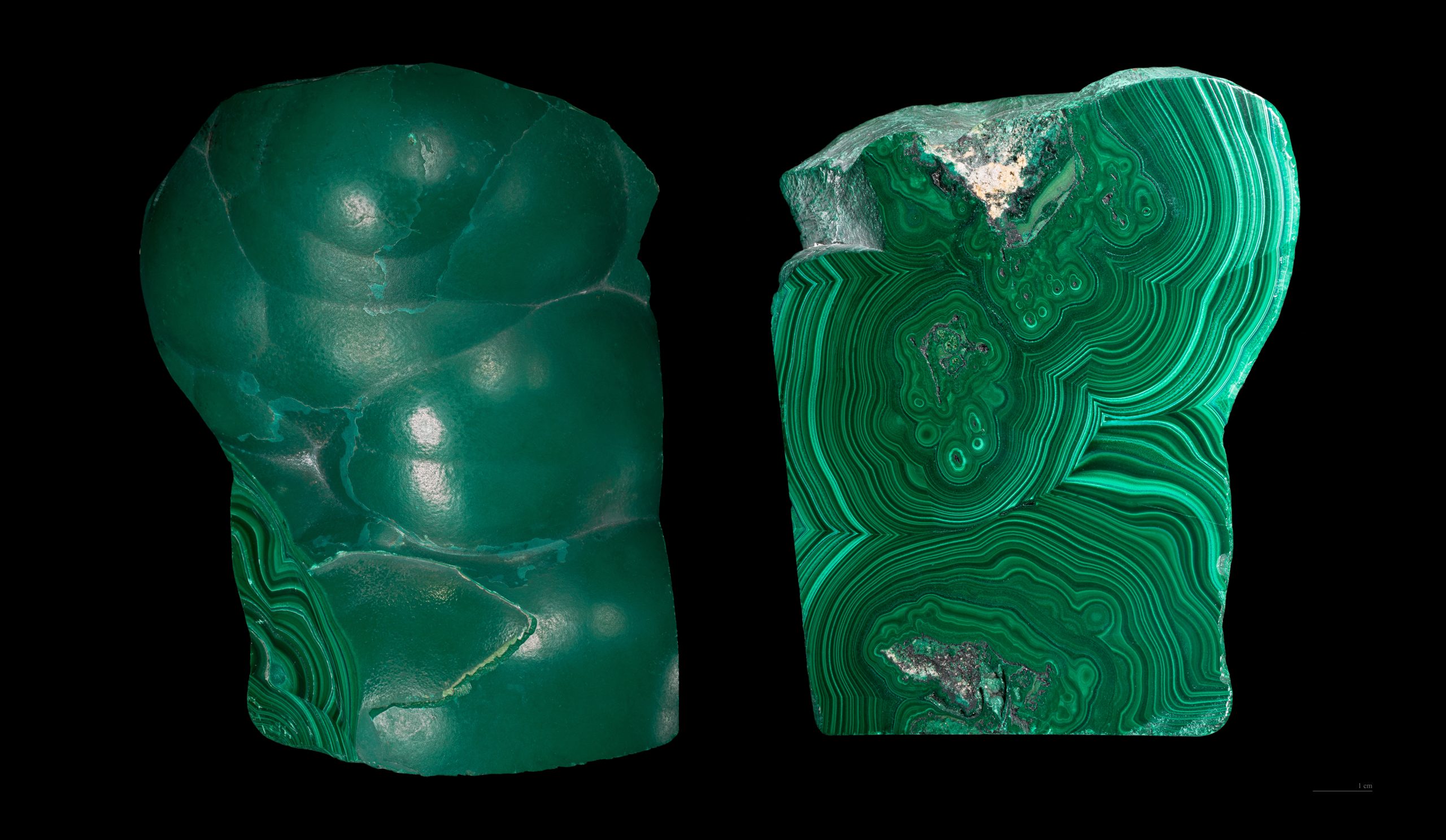 |
Hematite, malachite, smithsonite |
| Coating/laminae/druse Crystals that are small and coat surfaces |
 |
Quartz, calcite, malachite, azurite |
| Cubic Cube-shaped crystals |
 |
Pyrite, galena, halite |
| Dodecahedral 12-sided polygon shapes |
 |
Garnet, pyrite |
| Dendritic Branching crystals |
 |
Mn-oxides, copper, gold |
| Equant Crystals that do not have a long direction |
 |
Olivine, garnet, pyroxene |
| Fibrous Thin, very long crystals |
 |
Serpentine, amphibole, zeolite |
| Layered, sheets Stacked, very thin, flat crystals |
 |
Mica (biotite, muscovite, etc.) |
| Lenticular/platy Crystals that are plate-like |
 |
Selenite roses, wulfenite, calcite |
| Hexagonal Crystals with six sides |
 |
Quartz, hanksite, corundum |
| Massive/granular Crystals with no obvious shape, microscopic crystals |
 |
Limonite, pyrite, azurite, bornite |
| Octahedral 4-sided double pyramid crystals |
 |
Diamond, fluorite, magnetite, pyrite |
| Prismatic/columnar Very long, cylindrical crystals |
 |
Tourmaline, beryl, barite |
| Radiating Crystals that grow from a point and fan out |
 |
Pyrite “suns”, pyrophyllite |
| Rhombohedral Crystals shaped like slanted cubes |
 |
Calcite, dolomite |
| Tabular/blocky/stubby Sharp-sided crystals with no long direction |
 |
Feldspar, pyroxene, calcite |
| Tetrahedral 3-sided, pyramid-shaped crystals |
 |
Magnetite, spinel, tetrahedrite |
Table 3.4: Typical crystal habits of various minerals.


Another crystal habit that may be used to identify minerals is striations, which are dark and light parallel lines on a crystal face. Twinning is another, which occurs when the crystal structure replicates in mirror images along certain directions in the crystal.

Striations and twinning are related properties in some minerals including plagioclase feldspar. Striations are optical lines on a cleavage surface. Because of twinning in the crystal, striations show up on one of the two cleavage faces of the plagioclase crystal.
3.5.5 Cleavage and Fracture

Minerals often show characteristic patterns of breaking along specific cleavage planes or show characteristic fracture patterns. Cleavage planes are smooth, flat, parallel planes within the crystal. The cleavage planes may show as reflective surfaces on the crystal, as parallel cracks that penetrate into the crystal, or show on the edge or side of the crystal as a series of steps like rice terraces. Cleavage arises in crystals where the atomic bonds between atomic layers are weaker along some directions than others, meaning they will break preferentially along these planes. Because they develop on atomic surfaces in the crystal, cleavage planes are optically smooth and reflect light, although the actual break on the crystal may appear jagged or uneven. In such cleavages, the cleavage surface may appear like rice terraces on a mountainside that all reflect sunlight from a particular sun angle. Some minerals have a strong cleavage, some minerals only have weak cleavage or do not typically demonstrate cleavage.
For example, quartz and olivine rarely show cleavage and typically break into conchoidal fracture patterns.
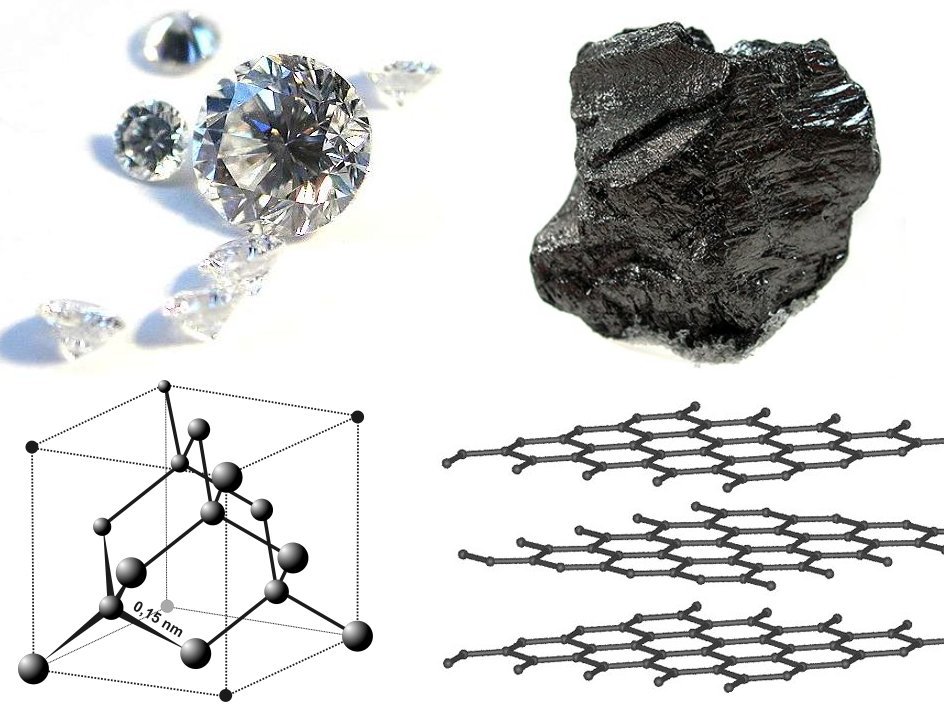
Graphite has its carbon atoms arranged into layers with relatively strong bonds within the layer and very weak bonds between the layers. Thus graphite cleaves readily between the layers and the layers slide easily over one another giving graphite its lubricating quality.

Mineral fracture surfaces may be rough and uneven or they may be show conchoidal fracture. Uneven fracture patterns are described as irregular, splintery, fibrous. A conchoidal fracture has a smooth, curved surface like a shallow bowl or conch shell, often with curved ridges. Natural volcanic glass, called obsidian, breaks with this characteristic conchoidal pattern.
To work with cleavage, it is important to remember that cleavage is a result of bonds separating along planes of atoms in the crystal structure. On some minerals, cleavage planes may be confused with crystal faces. This will usually not be an issue for crystals of minerals that grew together within rocks. The act of breaking the rock to expose a fresh face will most likely break the crystals along cleavage planes. Some cleavage planes are parallel with crystal faces but many are not. Cleavage planes are smooth, flat, parallel planes within the crystal. The cleavage planes may show as parallel cracks that penetrate into the crystal (see amphibole below), or show on the edge or side of the crystal as a series of steps like rice terraces. For some minerals characteristic crystal habit is to grow crystal faces even when surrounded by other crystals in rock. An example is garnet. Minerals grown freely where the crystals are unconstrained and can take characteristic shapes often form crystal faces (see quartz below).

In some minerals, distinguishing cleavage planes from crystal faces may be challenging for the student. Understanding the nature of cleavage and referring to the number of cleavage planes and cleavage angles on identification keys should provide the student with enough information to distinguish cleavages from crystal faces. Cleavage planes may show as multiple parallel cracks or flat surfaces on the crystal. Cleavage planes may be expressed as a series of steps like terraced rice paddies. See the cleavage surfaces on galena above or plagioclase below. Cleavage planes arise from the tendency of mineral crystals to break along specific planes of weakness within the crystal favored by atomic arrangements. The number of cleavage planes, the quality of the cleavage surfaces, and the angles between them are diagnostic for many minerals and cleavage is one of the most useful properties for identifying minerals. Learning to recognize cleavage is an especially important and useful skill in studying minerals.

As an identification property of minerals, cleavage is usually given in terms of the quality of the cleavage (perfect, imperfect, or none), the number of cleavage surfaces, and the angles between the surfaces. The most common number of cleavage plane directions in the common rock-forming minerals are: one perfect cleavage (as in mica), two cleavage planes (as in feldspar, pyroxene, and amphibole), and three cleavage planes (as in halite, calcite, and galena). One perfect cleavage (as in mica) develops on the top and bottom of the mineral specimen with many parallel cracks showing on the sides but no angle of intersection. Two cleavage planes intersect at an angle. Common cleavage angles are 60°, 75°, 90°, and 120°. Amphibole has two cleavage planes at 60° and 120°. Galena and halite have three cleavage planes at 90° (cubic cleavage). Calcite cleaves readily in three directions producing a cleavage figure called a rhomb that looks like a cube squashed over toward one corner giving rise to the approximately 75° cleavage angles. Pyroxene has an imperfect cleavage with two planes at 90°.

Cleavages on common rock-forming minerals:
- Quartz—none (conchoidal fracture)
- Olivine—none (conchoidal fracture)
- Mica—1 perfect
- Feldspar—2 perfect at 90°
- Pyroxene—2 imperfect at 90°
- Amphibole—2 perfect at 60°/120°
- Calcite—3 perfect at approximately 75°
- Halite, galena, pyrite—3 perfect at 90°
3.5.6 Special Properties

Special properties are unique and identifiable characteristics used to identify minerals or that allow some minerals to be used for special purposes. Ulexite has a fiber-optic property that can project images through the crystal like a high-definition television screen (see figure 3.61). A simple identifying special property is taste, such as the salty flavor of halite or common table salt (NaCl). Sylvite is potassium chloride (KCl) and has a more bitter taste.

Another property geologists may use to identify minerals is a property related to density called specific gravity. Specific gravity measures the weight of a mineral specimen relative to the weight of an equal volume of water. The value is expressed as a ratio between the mineral and water weights. To measure specific gravity, a mineral specimen is first weighed in grams then submerged in a graduated cylinder filled with pure water at room temperature. The rise in water level is noted using the cylinder’s graduated scale. Since the weight of water at room temperature is 1 gram per cubic centimeter, the ratio of the two weight numbers gives the specific gravity. Specific gravity is easy to measure in the laboratory but is less useful for mineral identification in the field than other more easily observed properties, except in a few rare cases such as the very dense galena or native gold. The high density of these minerals gives rise to a qualitative property called “heft.” Experienced geologists can roughly assess specific gravity by heft, a subjective quality of how heavy the specimen feels in one’s hand relative to its size.
A simple test for identifying calcite and dolomite is to drop a bit of dilute hydrochloric acid (10-15% HCl) on the specimen. If the acid drop effervesces or fizzes on the surface of the rock, the specimen is calcite. If it does not, the specimen is scratched to produce a small amount of powder and test with acid again. If the acid drop fizzes slowly on the powdered mineral, the specimen is dolomite. The difference between these two minerals can be seen in the video. Geologists who work with carbonate rocks carry a small dropper bottle of dilute HCl in their field kit. Vinegar, which contains acetic acid, can be used for this test and is used to distinguish non-calcite fossils from limestone. While acidic, vinegar produces less of a fizzing reaction because acetic acid is a weaker acid.

Video 3.2: Calcite and dolomite reacting with hydrochloric acid.
If you are using an offline version of this text, access this YouTube video via the QR code.

Some iron-oxide minerals are magnetic and are attracted to magnets. A common name for a naturally magnetic iron oxide is lodestone. Others include magnetite (Fe3O4) and ilmenite (FeTiO3). Magnetite is strongly attracted to magnets and can be magnetized. Ilmenite and some types of hematite are weakly magnetic.

Some minerals and mineraloids scatter light via a phenomenon called iridescence. This property occurs in labradorite (a variety of plagioclase) and opal. It is also seen in biologically created substances like pearls and seashells. Cut diamonds show iridescence and the jeweler’s diamond cut is designed to maximize this property.

Striations on mineral cleavage faces are an optical property that can be used to separate plagioclase feldspar from potassium feldspar (K-spar). A process called twinning creates parallel zones in the crystal that are repeating mirror images. The actual cleavage angle in plagioclase is slightly different than 90o and the alternating mirror images in these twinned zones produce a series of parallel lines on one of plagioclase’s two cleavage faces. Light reflects off these twinned lines at slightly different angles which then appear as light and dark lines called striations on the cleavage surface. Potassium feldspar does not exhibit twinning or striations but may show linear features called exsolution lamellae, also known as perthitic lineation or simply perthite. Because sodium and potassium do not fit into the same feldspar crystal structure, the lines are created by small amounts of sodium feldspar (albite) separating from the dominant potassium feldspar (K-spar) within the crystal structure. The two different feldspars crystallize out into roughly parallel zones within the crystal, which are seen as these linear markings.

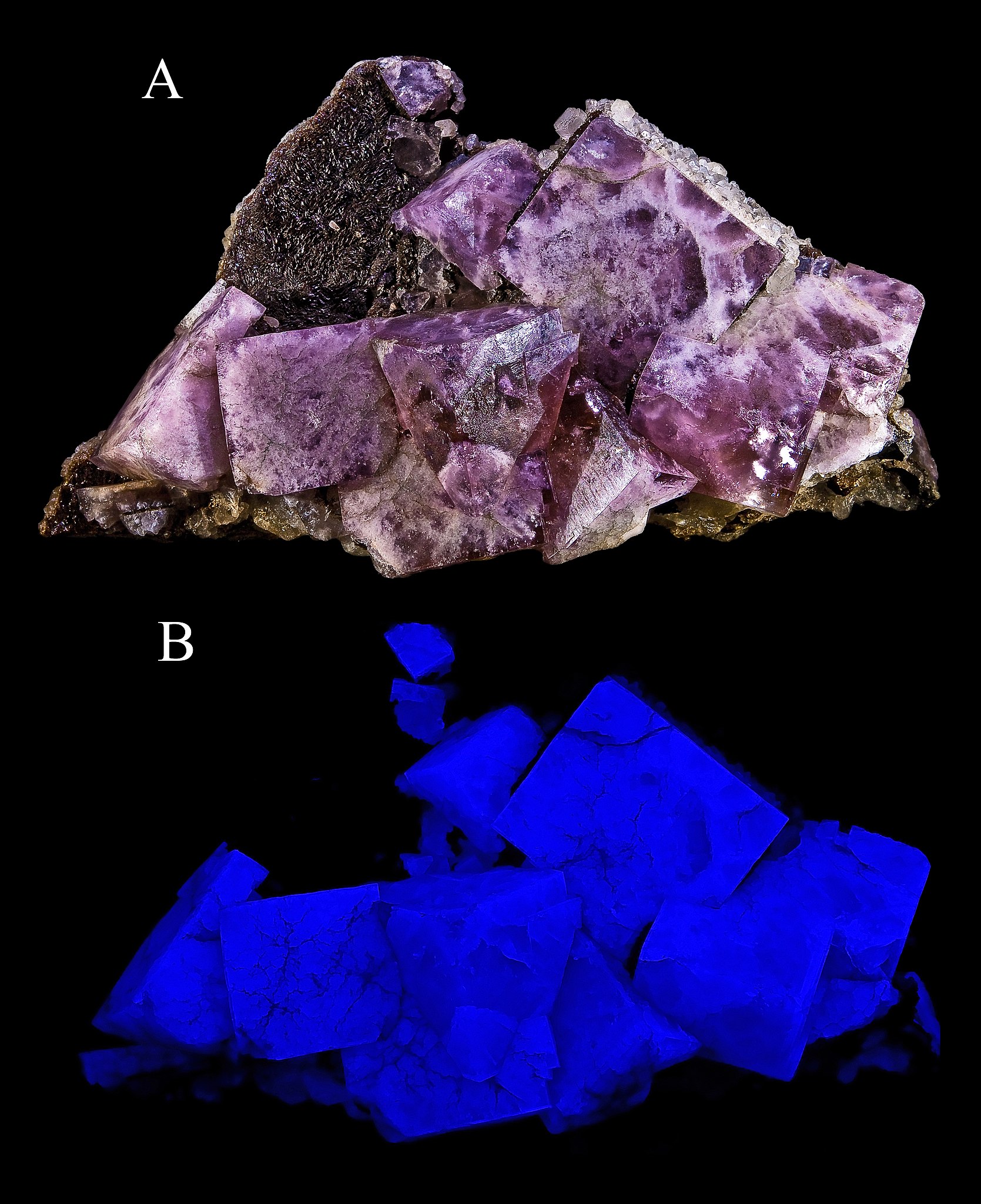
One of the most interesting special mineral properties is fluorescence. Certain minerals, or trace elements within them, give off visible light when exposed to ultraviolet radiation or black light. Many mineral exhibits have a fluorescence room equipped with black lights so this property can be observed. An even rarer optical property is phosphorescence. Phosphorescent minerals absorb light and then slowly release it, much like a glow-in-the-dark sticker.

Take this quiz to check your comprehension of this section.
If you are using an offline version of this text, access the quiz for section 3.5 via the QR code.
Summary
Minerals are the building blocks of rocks and essential to understanding geology. Mineral properties are determined by their atomic bonds. Most minerals begin in a fluid, and either crystallize out of cooling magma or precipitate as ions and molecules out of a saturated solution. The silicates are largest group of minerals on Earth, by number of varieties and relative quantity, making up a large portion of the crust and mantle. Based on the silicon-oxygen tetrahedra, the crystal structure of silicates reflects the fact that silicon and oxygen are the top two of Earth’s most abundant elements. Non-silicate minerals are also economically important, and providing many types of construction and manufacturing materials. Minerals are identified by their unique physical properties, including luster, color, streak, hardness, crystal habit, fracture, cleavage, and special properties.

Take this quiz to check your comprehension of this chapter.
If you are using an offline version of this text, access the quiz for chapter 3 via the QR code.
Text References
- Clarke, F.W.H.S.W., 1927, The Composition of the Earth’s Crust: Professional Paper, United States Geological Survey, Professional Paper.
- Gordon, L.M., and Joester, D., 2011, Nanoscale chemical tomography of buried organic-inorganic interfaces in the chiton tooth: Nature, v. 469, no. 7329, p. 194–197.
- Hans Wedepohl, K., 1995, The composition of the continental crust: Geochim. Cosmochim. Acta, v. 59, no. 7, p. 1217–1232.
- Lambeck, K., 1986, Planetary evolution: banded iron formations: v. 320, no. 6063, p. 574–574.
- Scerri, E.R., 2007, The Periodic Table: Its Story and Its Significance: Oxford University Press, USA.
- Thomson, J.J., 1897, XL. Cathode Rays: Philosophical Magazine Series 5, v. 44, no. 269, p. 293–316.
- Trenn, T.J., Geiger, H., Marsden, E., and Rutherford, E., 1974, The Geiger-Marsden Scattering Results and Rutherford’s Atom, July 1912 to July 1913: The Shifting Significance of Scientific Evidence: Isis, v. 65, no. 1, p. 74–82.
Figure References
Figure 3.1: The periodic table of the elements. R.A. Dragoset, A. Musgrove, C.W. Clark, and W.C. Martin — NIST, Physical Measurement Laboratory. 2010. Public domain. https://commons.wikimedia.org/wiki/File:Periodic_Table_-_Atomic_Properties_of_the_Elements.png
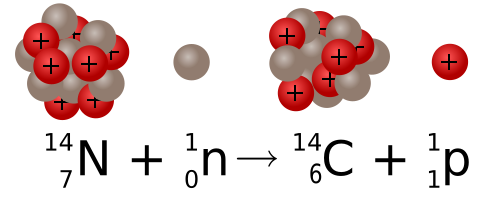
Figure 3.2: Formation of carbon-14 from nitrogen-14. Sgbeer; adapted by NikNaks. 2011. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Carbon_14_formation_and_decay.svg
Figure 3.3: Element abundance pie chart for Earth’s crust. Kindred Grey. 2022. CC BY 4.0.
Figure 3.4: A model of a water molecule, showing the bonds between the hydrogen and oxygen. Dan Craggs. 2009. Public domain. https://commons.wikimedia.org/wiki/File:H2O_2D_labelled.svg
Figure 3.5: The carbon dioxide molecule. Jynto. 2011. Public domain. https://commons.wikimedia.org/wiki/File:Carbon_dioxide_3D_ball.png
Figure 3.6: Cubic arrangement of Na and Cl in halite. Benjah-bmm27. 2006. Public domain. https://commons.wikimedia.org/wiki/File:Sodium-chloride-3D-ionic.png
Figure 3.7: Methane molecule. DynaBlast. 2006. CC BY-SA 2.5. https://commons.wikimedia.org/wiki/File:Covalent.svg
Figure 3.8: Calcium carbonate deposits from hard water. Bbypnda. 2014. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Hard_Water_Calcification.jpg
Figure 3.9: The Bonneville Salt Flats of Utah. Bureau of Land Management. 2015. CC BY 2.0. https://commons.wikimedia.org/wiki/File:Bonneville_Salt_Flats_(17423041595).jpg
Figure 3.10: Lava, magma at the Earth’s surface. Hawaii Volcano Observatory (DAS). 2003. Public domain. https://commons.wikimedia.org/wiki/File:Pahoehoe_toe.jpg
Figure 3.11: Ammonite shell made of calcium carbonate. Dlloyd. 2006. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Ammonite_Asteroceras.jpg
Figure 3.12: Rotating animation of a tetrahedra. Kjell André. 2005. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Tetrahedron.gif
Figure 3.13: Silicate tetrahedron. Helgi. 2013. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Silicate_tetrahedron_%2B.svg
Figure 3.14: Olivine crystals in basalt. Vsmith. 2005. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Peridot_in_basalt.jpg
Figure 3.15: Tetrahedral structure of olivine. Matanya (usurped). 2005. Public domain. https://commons.wikimedia.org/wiki/File:Atomic_structure_of_olivine_1.png
Figure 3.16: Crystals of diopside, a member of the pyroxene family. Robert M. Lavinsky. 2010. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Diopside-172005.jpg
Figure 3.17: Single chain tetrahedral structure in pyroxene. Bubenik. 2012. CC BY-SA 3.0. https://en.wikipedia.org/wiki/File:Pyroxferroite-chain.png
Figure 3.18: Elongated crystals of hornblende in orthoclase. Dave Dyet. 2007. Public domain. https://commons.wikimedia.org/wiki/File:Orthoclase_Hornblende.jpg
Figure 3.19: Hornblende crystals. Saperaud~commonswiki. 2005. Public domain. https://commons.wikimedia.org/wiki/File:Amphibole.jpg
Figure 3.20: Double chain structure. Bubenik. 2012. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Jimthompsonite-chain.png
Figure 3.21: Sheet crystals of biotite mica. Fred Kruijen. 2005. CC BY-SA 3.0 NL. https://commons.wikimedia.org/wiki/File:Biotite_aggregate_-_Ochtendung,_Eifel,_Germany.jpg
Figure 3.22: Crystal of muscovite mica. Saperaud~commonswiki. 2005. Public domain. https://commons.wikimedia.org/wiki/File:MicaSheetUSGOV.jpg
Figure 3.23: Sheet structure of mica. Benjah-bmm27. 2007. Public domain. https://en.wikipedia.org/wiki/File:Silicate-sheet-3D-polyhedra.png
Figure 3.24: Crystal structure of a mica. Rosarinagazo. 2006. Public domain. https://commons.wikimedia.org/wiki/File:Illstruc.jpg
Figure 3.25: Mica “silica sandwich” structure. Kindred Grey. 2022. CC BY 4.0. Includes Sandwich by Alex Muravev from Noun Project (Noun Project license).
Figure 3.26: Structure of kaolinite. USGS. Public domain. https://pubs.usgs.gov/of/2001/of01-041/htmldocs/clays/kaogr.htm
Figure 3.27: Freely growing quartz crystals showing crystal faces. JJ Harrison. 2009. CC BY-SA 2.5. https://en.wikipedia.org/wiki/File:Quartz,_Tibet.jpg
Figure 3.28: Mineral abundance pie chart in Earth’s crust. Kindred Grey. 2022. CC BY 4.0.
Figure 3.29: Pink orthoclase crystals. Didier Descouens. 2009. CC BY-SA 4.0. https://commons.wikimedia.org/wiki/File:OrthoclaseBresil.jpg
Figure 3.30: Crystal structure of feldspar. Taisiya Skorina and Antoine Allanore (DOI:10.1039/C4GC02084G). 2015. CC BY 3.0. https://www.researchgate.net/figure/fig1_273641498
Figure 3.31: Hanksite, Na22K(SO4)9(CO3)2Cl, one of the few minerals that is considered a carbonate and a sulfate. Matt Affolter(QFL247). 2009. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Hanksite.JPG
Figure 3.32: Calcite crystal in shape of rhomb. Note the double-refracted word “calcite” in the center of the figure due to birefringence. Alkivar. 2005. Public domain. https://commons.wikimedia.org/wiki/File:Calcite-HUGE.jpg
Figure 3.33: Limestone with small fossils. Jim Stuby. 2010. Public domain. https://commons.wikimedia.org/wiki/File:Limestone_etched_section_KopeFm_new.jpg
Figure 3.34: Bifringence in calcite crystals. Mikael Häggström. 2010. Public domain. https://commons.wikimedia.org/wiki/File:Positively_birefringent_material.svg
Figure 3.35: Crystal structure of calcite. Materialscientist. 2009. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Calcite.png
Figure 3.36: Limonite, a hydrated oxide of iron. USGS. 2005. Public domain. https://commons.wikimedia.org/wiki/File:LimoniteUSGOV.jpg
Figure 3.37: Oolitic hematite. Dave Dyet. 2007. Public domain. https://en.wikipedia.org/wiki/File:Hematite_-_oolitic_with_shale_Iron_Oxide_Clinton,_Oneida_County,_New_York.jpg
Figure 3.38: Halite crystal showing cubic habit. Saperaud~commonswiki. 2005. Public domain. https://en.wikipedia.org/wiki/File:ImgSalt.jpg
Figure 3.39: Salt crystals at the Bonneville Salt Flats. Michael. 2008. CC BY-SA 4.0. https://commons.wikimedia.org/wiki/File:Bonneville_salt_flats_pilot_peak.jpg
Figure 3.40: Fluorite. B shows fluorescence of fluorite under UV light. Didier Descouens. 2009. CC BY-SA 4.0. https://commons.wikimedia.org/wiki/File:FluoriteUV.jpg
Figure 3.41: Cubic crystals of pyrite. CarlesMillan. 2009. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:2780M-pyrite1.jpg
Figure 3.42: Gypsum crystal. USGS. 2004. Public domain. https://commons.wikimedia.org/wiki/File:SeleniteGypsumUSGOV.jpg
Figure 3.43: Apatite crystal. Didier Descouens. 2010. CC BY-SA 4.0. https://commons.wikimedia.org/wiki/File:Apatite_Canada.jpg
Figure 3.44: Native sulfur deposited around a volcanic fumarole. Brisk g. 2006. Public domain. https://commons.wikimedia.org/wiki/File:Fumarola_Vulcano.jpg
Figure 3.45: Native copper. Jonathan Zander (Digon3); adapted by Materialscientist. 2009. CC BY-SA 2.5. https://commons.wikimedia.org/wiki/File:NatCopper.jpg
Figure 3.46: The rover Curiosity drilled a hole in this rock from Mars, and confirmed the mineral hematite, as mapped from satellites. NASA/JPL-Caltech/MSSS. Public domain. https://www.nasa.gov/jpl/msl/pia19036/
Figure 3.47: 15 mm metallic hexagonal molybdenite crystal from Quebec. John Chapman. 2008. CC BY-SA 4.0. https://commons.wikimedia.org/wiki/File:Molly_Hill_molybdenite.JPG
Figure 3.48: Submetallic luster shown on an antique pewter plate. Unknown author. ca. 1770 and 1810. Public domain. https://commons.wikimedia.org/wiki/File:Pewter_Plate.jpg
Table 3.3: Nonmetallic luster descriptions and examples. Quartz Brésil by Didier Descouens, 2010 (CC BY-SA 4.0, https://commons.wikimedia.org/wiki/File:Quartz_Br%C3%A9sil.jpg). KaolinUSGOV by Saperaud~commonswiki, 2005 (Public domain, https://commons.wikimedia.org/wiki/File:KaolinUSGOV.jpg). Selenite Gips Marienglas by Ra’ike, 2006 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Selenite_Gips_Marienglas.jpg). Mineral Mica GDFL006 by Luis Miguel Bugallo Sánchez, 2005 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Mineral_Mica_GDFL006.JPG). Sphalerite4 by Andreas Früh, 2005 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Sphalerite4.jpg).
Figure 3.49: Azurite is ALWAYS a dark blue color, and has been used for centuries for blue pigment. Graeme Churchard. 2013. CC BY 2.0. https://en.wikipedia.org/wiki/File:Azurite_in_siltstone,_Malbunka_mine_NT.jpg
Figure 3.50: Different minerals may have different streaks. Ra’ike. 2010. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Streak_plate_with_Pyrite_and_Rhodochrosite.jpg
Figure 3.51: Mohs hardness scale. NPS. Public domain (full license here). https://www.nps.gov/articles/mohs-hardness-scale.htm
Table 3.4: Typical crystal habits of various minerals. Elbaite-Lepidolite-Quartz-gem7-x1a by Robert M. Lavinsky, 2010 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Elbaite-Lepidolite-Quartz-gem7-x1a.jpg). Pyrophyllite-290575 by Robert M. Lavinsky, 2010 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Pyrophyllite-290575.jpg). Kyanite crystals by Aelwyn, 2006 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Kyanite_crystals.jpg). Malachite Kolwezi Katanga Congo by Didier Descouens, 2012 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Malachite_Kolwezi_Katanga_Congo.jpg). Ametyst-geode by Juppi66, 2009 (Public domain, https://commons.wikimedia.org/wiki/File:Ametyst-geode.jpg). Calcite-Galena-elm56c by Robert M. Lavinsky, before March 2010 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Calcite-Galena-elm56c.jpg). Pyrite elbe by Didier Descouens, 2011 (CC BY-SA 4.0, https://commons.wikimedia.org/wiki/File:Pyrite_elbe.jpg). Dendrites01 by Wilson44691, 2008 (Public domain, https://commons.wikimedia.org/wiki/File:Dendrites01.jpg). Peridot2 by S kitahashi, 2006 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Peridot2.jpg). Tremolite Campolungo by Didier Descouens, 2009 (CC BY-SA 4.0, https://en.wikipedia.org/wiki/File:Tremolite_Campolungo.jpg). Muscovite-Albite-122887 by Robert M. Lavinsky, 2010 (CC BY-SA 3.0, https://en.wikipedia.org/wiki/File:Muscovite-Albite-122887.jpg). Calcite-Wulfenite-tcw15a by Robert M. Lavinsky, 2010 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Calcite-Wulfenite-tcw15a.jpg). Hanksite by Matt Affolter(QFL247), 2009 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Hanksite.JPG). LimoniteUSGOV by USGS, unknown date (Public domain, https://en.wikipedia.org/wiki/File:LimoniteUSGOV.jpg). Fluorite crystals 270×444 by Ryan Salsbury, 2004 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Fluorite_crystals_270x444.jpg). Calcite-HUGE by Alkivar, 2005 (Public domain, https://commons.wikimedia.org/wiki/File:Calcite-HUGE.jpg). Diopside-172005 by Robert M. Lavinsky, before March 2010 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Diopside-172005.jpg). Tetrahedrite-Chalcopyrite-Sphalerite-251531 by Robert M. Lavinsky, before March 2010 (CC BY-SA 3.0, https://commons.wikimedia.org/wiki/File:Tetrahedrite-Chalcopyrite-Sphalerite-251531.jpg).
Figure 3.52: Gypsum with striations. Didier Descouens. 2009. CC BY-SA 4.0. https://commons.wikimedia.org/wiki/File:Gypse_Caresse.jpg
Figure 3.53: Twinned staurolite. Virginia State Parks. 2013. CC BY 2.0. https://flic.kr/p/WxNWqi
Figure 3.54: Striations on plagioclase. Mike Beauregard. 2011. CC BY 2.0. https://flic.kr/p/9xh4MS
Figure 3.55: Citrine, a variety of quartz showing conchoidal fracture. James St. John. 2021. CC BY 2.0. https://flic.kr/p/2ky61rb
Figure 3.56: Photos and crystal structures of diamond as compared to graphite. Itub; adapted by Materialscientist. 2009. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Diamond_and_graphite2.jpg
Figure 3.57: Cubic cleavage of galena; note how the cleavage surfaces show up as different but parallel layers in the crystal. Modris Baum. 2012. Public domain. https://commons.wikimedia.org/wiki/File:Argentiferous_Galena-458851.jpg
Figure 3.58: Freely growing quartz crystals showing crystal faces. JJ Harrison. 2009. CC BY-SA 2.5. https://en.wikipedia.org/wiki/File:Quartz,_Tibet.jpg
Figure 3.59: Steps of cleavage along the same cleavage direction. USGS; adapted by David Remahl. 2004. Public domain. https://commons.wikimedia.org/wiki/File:WollastoniteUSGOV.jpg
Figure 3.60: Photomicrograph showing 120/60 degree cleavage within a grain of amphibole. Eurico Zimbres. 1990. CC BY-SA 2.5. https://en.wikipedia.org/wiki/File:Amphibol.jpg
Figure 3.61: A demonstration of ulexite’s image projection. Dave Merrill. 2005. CC BY-SA 2.0. https://commons.wikimedia.org/wiki/File:Ulexite_on_flickr_%2821734610%29.jpg
Figure 3.62: Native gold has one of the highest specific gravities. Gump Stump. 2008. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Latrobe_gold_nugget_Natural_History_Museum.jpg
Figure 3.63: Paperclips attach to lodestone (magnetite). Ryan Somma. 1980. CC BY-SA 2.0. https://commons.wikimedia.org/wiki/File:Magnetite_Lodestone.jpg
Figure 3.64: Iridescence on plagioclase, also showing striations on the cleavage surface. Mike Beauregard. 2011. CC BY 2.0. https://flic.kr/p/9xh4MS
Figure 3.65: Exsolution lamellae within potassium feldspar. Jstuby. 2009. Public domain. https://commons.wikimedia.org/wiki/File:Perthitic_feldspar_Dan_Patch_SD.jpg
Figure 3.66: Fluorite. B shows fluorescence of fluorite under UV light. Didier Descouens. 2009. CC BY-SA 4.0. https://commons.wikimedia.org/wiki/File:FluoriteUV.jpg
A natural substance that is typically solid, has a crystalline structure, and is typically formed by inorganic processes. Minerals are the building blocks of most rocks.
Two or more atoms or ions that are connected chemically.
A anion structure of one silicon bonded to four oxygens, in the shape of a tetrahedron, with the silicon in the center and four oxygens at the corners of the structure. It has a net charge of -4, and can bond to cations to form silicate minerals.
Mineral group in which the silica tetrahedra, SiO4-4, is the building block.
Minerals in which ions are bonded to oxygen, such as hematite (Fe2O3).
Minerals bonded via a sulfur (S-2) atom.
Minerals bonded via a sulfate ion, SO4-2.
Mineral group in which the carbonate ion, CO3-2, is the building block. This can also refer to the rocks that are made from these minerals, namely limestone and dolomite (dolostone).
The measure of the vibrational (kinetic) energy of a substance.
The mineral makeup of a rock, i.e. which minerals are found within a rock.
CaCO3. Pure form is clear, but can take on many different colors with impurities. It is soft, fizzes in acid, and has three cleavages that are not at 90°.
A group of all atoms with a specific number of protons, having specific, universal, and unique properties.
Changes in sedimentary rocks due to increased (but low when compared to metamorphism) temperatures and pressures. This can include deposition of new minerals (e.g. limestone converting to dolomite) or dissolution of existing minerals.
Former swamp-derived (plant) material that is part of the rock record.
Dark colored volcanic glass, with extremely small microscopic crystals or no crystals. Typically form from felsic volcanism.
Rocks that are formed from liquid rock, i.e. from volcanic processes.
The physical breakdown (weathering) of bedrock by processes such as pressure, ice expansion, etc.
Breaking down of mineral material via chemical methods, like dissolution and oxidation.
The act of a solid coming out of solution, typically resulting from a drop in temperature or a decrease of the dissolving material.
The act of taking a solid and dissolving it into a liquid. This commonly occurs with salts and other minerals in water.
Rocks and minerals that change within the Earth are called metamorphic, changed by heat and pressure. Metamorphism is the name of the process.
An atom that has different number of neutrons but the same number of protons. While most properties are based on the number of protons in an element, isotopes can have subtle changes between them, including temperature fractionation and radioactivity.
The layers of igneous, sedimentary, and metamorphic rocks that form the continents. Continental crust is much thicker than oceanic crust. Continental crust is defined as having higher concentrations of very light elements like K, Na, and Ca, and is the lowest density rocky layer of Earth. Its average composition is similar to granite.
An atom or molecule that has a charge (positive or negative) due to the loss or gain of electrons.
(Fe,Mg)2SiO4. Typically translucent olive green and equant, with no cleavage. Common in mafic igneous rocks and in the mantle, but easily weathered in surface conditions. Structure is isolated silica tetrahedra. Known as peridot when a gem.
A rule that says the outer valence shell of electrons is complete when it contains 8 electrons.
A positively-charged ion. In geology, this commonly includes ions of the elements Ca+2, Na+1, K+1, Fe+2,+3, Al+3, and Mg+2.
A negatively-charged ion. In geology, this commonly includes elements and molecules like SiO4-4, S-2, SO4-4, and O-2.
Certain metallic elements (like iron) take in oxygen, causing reactions like rust.
Also known as rock salt, or table salt. 3 cleavages at 90°, cubic crystal habit. Typically clear or white, hardness of 3.
The process of liquid rock solidifying into solid rock. Because liquid rock is made of many components, the process is complex as different components solidify at different temperatures.
Liquid rock within the Earth.
The process in which solids (like minerals) are disassociated and the ionic components are dispersed in a liquid (usually water).
A solution that has the maximum allowed dissolved component, and is unable to dissolve more.
Pieces of rock that have been weathered and possibly eroded.
Porous variety of carbonate that form in relatively unheated water, sometimes as towers and spires.
A period of cooler temperatures on Earth in which ice sheets can grow on continents.
Porous, concentric, or layered variety of carbonate that forms with often heated water in springs and/or caves.
An elevated erosional surface caused by glacial or fluvial action.
A place where pressurized groundwater flows onto the surface.
A channelled body of water.
Liquid rock on the surface of the Earth.
Minerals with the same composition and different crystal structures.
Places that are under ocean water at all times.
Relatively flat ocean floor, which accumulates very fine grained detrital and chemical sediments.
Rocks that are formed by sedimentary processes, including sediments lithifying and precipitation from solution.
A chemical or biochemical rock made of mainly calcite.
A very fine grained version of silica deposited with or without microfossils.
An animal that possesses a spinal column or backbone.
Minerals that are bonded with the phosphate anion, PO4+3.
Any evidence of ancient life.
Middle chemical layer of the Earth, made of mainly iron and magnesium silicates. It is generally denser than the crust (except for older oceanic crust) and less dense than the core.
The outermost chemical layer of the Earth, defined by its low density and higher concentrations of lighter elements. The crust has two types: continental, which is the thick, more ductile, and lowest density, and oceanic, which is higher density, more brittle, and thinner.
An intrusive ultramafic rock, which is the main component of the mantle. The minerals in peridotite are typically olivine with some pyroxene.
General name of a mafic rock that is extrusive. Generally has a black groundmass color.
Two or more elements that can easily substitute for each other, due to similarities in ionic size and charge.
Can refer to a volcanic rock with lower silica composition, or the minerals that make up those rocks, namely olivine, pyroxene, amphibole, and biotite. Mafic rocks are darker in color and contain more minerals that are dark in color, but can contain some plagioclase feldspar. Primary mafic rocks are basalt (extrusive) and gabbro (intrusive).
The theory that the outer layer of the Earth (the lithosphere) is broken in several plates, and these plates move relative to one another, causing the major topographic features of Earth (e.g. mountains, oceans) and most earthquakes and volcanoes.
A solid part of the lithosphere which moves as a unit, i.e. the entire plate generally moves the same direction at the same speed.
A process where an oceanic plate descends bellow a less dense plate, causing the removal of the plate from the surface. Subduction causes the largest earthquakes possible, as the subducting plate can lock as it goes down. Volcanism is also caused as the plate releases volatiles into the mantle, causing melting.
ZrSiO4. Relatively chemically inert with a hardness of 8.5. Common accessory mineral in igneous and metamorphic rocks, as well as detrital sediments. Uranium can substitute for zirconium, making zircon a valuable mineral in radiometric dating.
A qualitative measure of the amount of metamorphism that has occurred or the amount of a resource present in an ore.
XY(Al,Si)2O6, in which X typically equals Na, Ca, Mg, or Fe and Y typically equals Mg, Fe, or Al. Typically black to dark green, blocky, with two cleavages at ~90°. Common in mafic igneous rocks and some metamorphic rocks. Structure is a single chain of silica tetrahedra.
General name of a mafic rock that is intrusive. Has more mafic minerals than felsic minerals.
Rock more metamorphosed than phyllite, to the point that mica grains are visible. Larger porphyroblasts are sometimes present.
A group of chain silicate minerals that form needlelike or prismatic crystals. Can be many colors but the most common form, hornblende, is dark brown to black. Has oblique cleavages at 54° and 126°. Common in many igneous rocks and some metamorphic rocks.
The typical form or forms a crystal takes when it grows.
Rocks formed via heat and/or pressure which change the minerals within the rock.
General name of an intermediate rock that is intrusive. Has about the same amount of felsic minerals and mafic minerals.
General name of a felsic rock that is intrusive. Has more felsic minerals than mafic minerals.
X1A2-3Z4O10(OH, F)2, where commonly X=K, Na, Ca; A=Al, Mg, Fe; Z=Si, Al. Has two more-common occurrences, light-colored (translucent and pearly tan) muscovite, and dark-colored biotite. Has one strong cleavage, and is typically seen as sheets, in stacks or "books." Common in many igneous and metamorphic rocks. Structure is two-dimensional sheets of silica tetrahedra in a hexagonal network.
Can refer to a volcanic rock with higher silica composition, or the minerals that make up those rocks, namely quartz, feldspar, and muscovite mica. Felsic rocks are lighter in color and contain more minerals that are light in color. Primary felsic rocks are rhyolite (extrusive) and granite (intrusive).
Consisting of three end members: potassium feldspar (K-spar, KAlSi3O8), plagioclase with calcium (CaAl2Si2O8, called anorthite), and plagioclase with sodium (NaAlSi3O8, called albite). Commonly blocky, with two cleavages at ~90°. Plagioclase is typically more dull white and gray, and K-spar is more vibrant white, orange, or red.
Rock spire that is offshore and a remnant of a rock layer.
Breaking down rocks into small pieces by chemical or mechanical means.
SiO2. Transparent, but can be any color imaginable with impurities. No cleavage, hard, and commonly forms equant masses. Perfect crystals are hexagonal prisms topped with pyramidal shapes. One of the most common minerals, and is found in many different geologic settings, including the dominant component of sand on the surface of Earth. Structure is a three-dimensional network of silica tetrahedra, connected as much as possible to each other.
The thin, outer layer of the Earth which makes up the rocky bottom of the ocean basins. Oceanic crust is much thinner (but denser) than continental crust. Oceanic crust is made of rocks similar to basalt and as it cools, becomes more dense.
General name of a felsic rock that is extrusive. Generally has a white, tan, or pink groundmass color.
Sedimentary rocks made of mineral grains weathered as mechanical detritus of previous rocks, e.g. sand, gravel, etc.
Minerals with a luster similar to metal and contain metals, including valuable elements like lead, zinc, copper, tin, etc.
The process of turning sediment into sedimentary rock, including deposition, compaction, and cementation.
Waves that change direction due to changing speeds, typically caused by a change in density of the medium.
An acid that forms from carbon dioxide and water. It is a large contributor to chemical weathering.
An evaporite mineral, CaSo4•2H2O. Has one cleavage, hardness of 2. Typically clear or white.
A chemical sedimentary rock that forms as water evaporates.
Minerals based on bonds to column 17 halogens, such as chlorine and fluorine.
Valuable material in the Earth, typically used for metallic mineral resources.
A rock primarily made of sand.
A feature with no internal structure, habit, or layering.
A specific chemical composition that forms different minerals and different temperatures and pressures. Quartz has several different polymorphs, including coesite, tridimite, and stishovite.
A highly weathered soil deposit that consists of aluminum ores.
Place where material is extracted from the Earth for human use.
Minerals made from just a single element, bonded to itself. Examples include gold, silver, copper, and diamond, which is a native version of carbon.
The shine a mineral takes on, based on the way light reflects off of a mineral. This is typically divided into two main categories: metallic (metal-like shine) and nonmetallic (non metal-like shine).
The color(s) that a mineral produces when powdered or rubbed against a hard surface, usually a porcelain tile.
The ease or difficulty in scratching a mineral, measured by the qualitative Mohs hardness scale, which ranges from soft talc (#1 on the scale) to hard diamond (#10 on the scale).
A weakness within the atomic structure of a mineral, which allows the mineral to break more easily along that plane. Minerals can have one, two, three, or more cleavages. Cleavage can also refer to the alignment of features within metamorphic rocks., though they are unrelated.
A break within a rock that has no relative movement between the sides. Caused by cooling, pressure release, tectonic forces, etc.
Minerals that have a luster that is not similar to metal. Divided into subtypes based on the way light reflects (or doesn't), including glassy/vitreous, greasy, pearly, dull, etc.
A mineral that shows no crystal habit, either because it is not prone to have a habit, or because it grew in a way that it was confined so it could not grow with its normal habit.
A mineral which only shows some characteristics of its true crystal habit, and is not perfectly grown.
A mineral that perfectly shows its true crystal habit.
Fractures that have a circular appearance.
Place where lava is erupted at the surface.
Related to density; the ratio of the weight of a mineral vs. the weight of an equal volume of water.
An observation which is based on non-numerical data. While these types of observations are not preferred, they can still be useful.
An observation which is influenced by the observer's personal bias.
Linear alignment of minerals within a rock.

