2 Basic Laboratory Measurements
Learning Objectives
- Evaluate clinical data to determine pathways involved in metabolic homeostasis.
- Recognize the utility of values for blood lactate, urinalysis, ALT/AST, and lipid profiles in clinical decision making.
- Determine how enzymes are regulated by comparing the role of inhibitors and activators, with examples, including: transition state inhibitors, irreversible inhibitors, and competitive and noncompetitive inhibitors/activators (see section 1.2).
- Identify vitamins as cofactors and necessary components of biological systems and begin to associate common symptoms with nutritional deficiencies.
About this Chapter
The cell is the most fundamental unit of all eukaryotic organisms. Its components and their cellular interactions are essential to the inner workings of the human body. Cells are primarily influenced by:
- the surrounding environment,
- through cell-cell interactions, and
- through circulating signals or hormones.
As a clinician, your first indication of changes to these cellular components will be illustrated by the signs and symptoms of your patient. Following this generalized assessment, you will begin to dissect out a clinical diagnosis by interpreting basic lab values. Each of these elements are indicative of molecular changes ultimately leading to the presentation you are challenged with.
2.1 Laboratory Values and Biochemical Correlates
A comprehensive metabolic panel (CMP) is a blood test that measures a variety of compounds (such as blood glucose and electrolytes), and it can be used to determine fluid balance, kidney function, and liver function, as well as other key metabolic functions. It is often used to determine health status (or metabolic dysfunction) and gives you insight into changes in biochemical reactions. Other diagnostics such as a complete blood count (CBC) may also be used but will not be discussed here.
How to read a CMP both clinically and biochemically will help hone the skills of diagnosis and maintenance of health status in patients. Additional laboratory tests such as a lipid profile, blood lactate, or urinalysis may also be ordered to supplement information from the CMP.
Deviations in any of these values can help determine changes in substrate availability, cofactors, and vitamin or enzymatic deficiencies. It will also help you better understand how biochemical pathways can influence clinical signs and symptoms.
Comprehensive metabolic panel
A CMP is often administered as part of a routine physical exam or for monitoring of specific conditions that impact kidney and liver functions. The results include the following tests (table 2.1):
| Analyte | Normal range |
|---|---|
| Chemistries | |
| Glucose | 70–100 mg/dL |
| Calcium | 8.4–10.5 mg/dL |
| Protein | |
| Total protein | 6.1–8.0 g/dL |
| Albumin | 3.2–4.6 g/dL |
| Electrolytes | |
| Sodium | 135-144 mmol/L |
| Potassium | 3.6–5.0 mmol/L |
| Chloride | 101–111 mmol/L |
| CO2 | 21–35 mmol/L |
| Kidney tests | |
| Blood urea nitrogen (BUN) | 5–20 mg/dL |
| Creatinine | 0.44–1.00 mg/dL |
| Liver tests | |
| Alkaline phosphatase (ALP) | 38–126 IU/L |
| Aspartate amino transferase (AST) | 14.9–39.9 IU/L |
| Alanine amino transferase (ALT) | 11–43 IU/L |
| Bilirubin, total | 0.0–1.2 mg/dL |
| Globulin | 1.5–3.5 g/dL |
| Direct bilirubin | <0.3 mg/dL |
Table 2.1: Normal values for a typical comprehensive metabolic panel. These values will be given to you when evaluating information.
Glucose - This energy source for the body is maintained in a very narrow range. Metabolic pathways are in place to balance both glucose uptake and glucose output to keep this value constant. Glucose homeostasis is regulated hormonally, and deviations from normal values could suggest metabolic or hormonal deficiencies (chapter 4 and chapter 5).
Calcium - This is one of the most important minerals in the body; it is essential for the proper functioning of muscles, nerves, and cardiac tissue. It is a cofactor in processes such as blood clotting and bone formation. Other vitamins also play key roles in these pathways (vitamin K in clotting and vitamin D in bone formation), so understanding this value may give insights into other potential deficiencies.
Proteins
Albumin - Albumin is a major serum protein produced in the liver and is a nonspecific carrier of many lipid soluble vitamins and other hydrophobic compounds. It is also essential for maintaining oncotic pressure. Decreases in serum albumin may be suggestive of nutritional deficiencies or changes in plasma volume as well as poor liver function. Therefore accessibility of lipid soluble vitamins, minerals, and hormones may be diminished secondarily to a decrease in albumin.
Total protein - Like serum albumin, a measure of total serum protein is useful to evaluate malnutrition or more chronic disorders such as inflammatory bowel disease. Increased production of immunoglobulins could also be detected here and would be indicative of chronic illness.
Electrolytes
Sodium - Sodium is vital to normal body processes, including nerve and muscle function. Hyponatremia can be suggestive of illness, diarrhea, or malnutrition, while hypernatremia is most often caused by an increased loss of water (dehydration) potentially due to endocrine disorders such as Cushing syndrome or diabetes insipidus.
Potassium - Potassium is critical for cardiac function, and although hypo or hyperkalemia can be indicative of a variety of disorders, it can be a critical indicator of maintenance of diabetes. Unmanaged diabetic individuals may present with hyperkalemia, however, inappropriate insulin administration will increase potassium uptake. Therefore poor management can cause a sudden drop in potassium (hypokalemia) leading to cardiac dysfunction.
CO2 (carbon dioxide, bicarbonate) - CO2 is produced from several oxidative pathways and is removed in the form of bicarbonate or through hemoglobin transport. Elevation of CO2 could suggest a renal, respiratory, and/or metabolic concern, and additional laboratory values would need to be assessed to determine the root cause. These may include blood lactate, blood urea nitrogen (BUN), as well as arteriole blood gasses (ABG).
Chloride - Chloride is a negatively charged ion that works with other electrolytes (potassium, sodium, and bicarbonate) to help regulate both fluid and acid–base (pH) balance in the body. Chloride and electrolyte tests may help diagnose the cause of signs and symptoms such as prolonged vomiting, diarrhea, weakness, and difficulty breathing (respiratory distress).
Kidney tests
Blood urea nitrogen (BUN) - Urea is a waste product of amino acid metabolism filtered out of the blood by the kidneys. It is a primary means of nitrogen disposal, and conditions that affect the kidneys have the potential to affect the amount of urea in the blood. This value is also indicative of deficiencies in amino acid metabolism, or changes in urea cycle activity or protein catabolism (section 5.3).
Creatinine - This waste product is produced in the muscles and filtered out by the kidneys. Urinary levels of creatinine are a good indicator of how the kidneys are working.
Liver tests
Alkaline phosphatase (ALP) - ALP is an enzyme found in the liver and other tissues such as bone. Elevated levels of ALP are most commonly caused by liver disease or other pathologies that increase cell damage leading to the release of ALP in the blood. Other disorders that impact bone growth may also increase ALP.
Alanine amino transferase (ALT) - ALT is an enzyme found predominantly in the liver and kidney. It is important in movement of ammonia (through the process of transamination) in tissues, and an elevation of ALT in circulation suggests liver damage (or potentially muscle damage) (section 5.3).
Aspartate amino transferase (AST) - AST is also a transferase needed in nitrogen metabolism found especially within the heart and liver. It is also a useful test for detecting liver damage. The ratio of ALT/AST can be used to distinguish between disorders such as alcoholic versus nonalcoholic fatty liver disease (section 5.3).
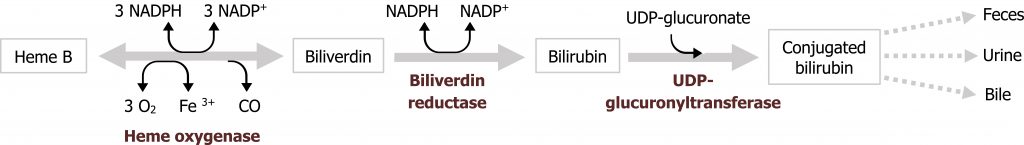
Bilirubin - Bilirubin is a waste product produced by the degradation of heme. Heme degradation within the liver is a normal part of red blood cell turnover, but elevated bilirubin could also be indicative of excessive hemolysis (due to deficiencies in NAPDH or increased oxidative stress) or biliary obstructions. Bilirubin values can be reported as direct (conjugated) or indirect (unconjugated) bilirubin. As conjugation takes place in the liver, decreased conjugated bilirubin or increased unconjugated bilirubin would suggest liver dysfunction (figure 2.1).
Lipid profile
A lipid profile (table 2.2) is often used to assess risk of developing cardiovascular disease (CVD) or to monitor the effectiveness of a dietary or pharmacological intervention.
| Serum measurement | Desirable levels |
|---|---|
| Total cholesterol | <200 mg/dL |
| Low-density lipoprotein cholesterol (LDL-C) | <100 mg/dL |
| High-density lipoprotein cholesterol (HDL-C) | over 40 mg/dL for females; over 60 mg/dL for males |
| Triglycerides (TGs) | <150 mg/dL |
| TG to HDL ratio (calculated) | <5 |
Table 2.2: Desirable (optimal) values for lipids. Ranges of intermediate and high can also be found for these values.
Total cholesterol - This measurement takes in to account various forms of cholesterol in circulation. It is the total of high-density lipoprotein (HDL), low-density lipoprotein (LDL), and 20 percent of the triglyceride measurement. This is key to determining your cholesterol ratio (total/HDL), which should be below 5 with an ideal ratio being 3.5.
High-density lipoprotein cholesterol (HDL-C) - HDL is predominantly involved in reverse cholesterol transport because it removes excess cholesterol from peripheral tissues and carries it to the liver for removal or use. It has several key interactions with very low-density lipid (VLDL) particles in circulation that assist in lipid metabolism.
Low-density lipoprotein cholesterol (LDL-C) - LDL is often called "bad cholesterol" because it can deposit excess cholesterol in walls of blood vessels, which can contribute to atherosclerosis.
Triglycerides - This is a measurement of circulating triacylglycerols (TAG), which are primarily transported by VLDL particles. TAG levels should be less than 150 mg/dL, and increased TAG may suggest endocrine deficiencies or metabolic defects.
Variations of normal in a lipid profile could be suggestive of heritable disorders, poor diet, or lipid uptake, decreased lipid storage, or excessive synthesis. The combination of these values will help determine what aspect of lipid metabolism is altered (chapter 6).
Lactate
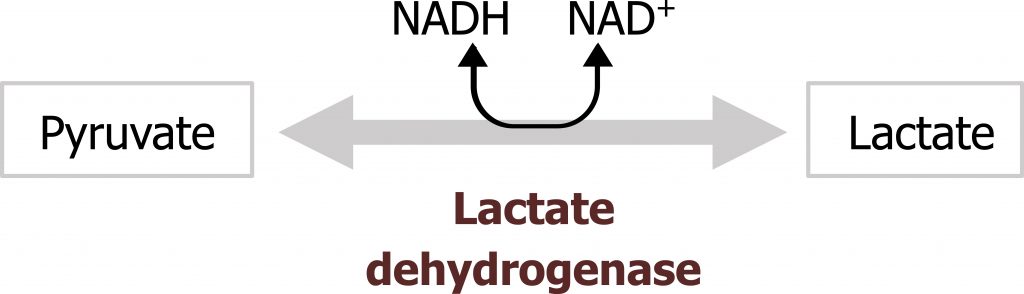
Serum lactate levels may also be measured in conjunction with a complete metabolic panel. Serum lactate should be negligible under normal conditions, however, elevated lactate could be suggestive of excessive anaerobic metabolism, such as is the case in intense exercise or deficiency in oxygen transport caused by ischemic injury. This could also be caused by inappropriate diversion of substrate such as is the case in some enzymatic deficiencies (pyruvate dehydrogenase deficiency) or changes in NADH levels (figure 2.2).
Urinalysis (includes a visual, chemical, and microscopic exam)
Visual exam and the microscopic exam
Although both the visual and microscopic exam are very essential components to this analysis, these will not be focused on here. The color of urine can vary, most often shades of yellow, from very pale or colorless to very dark or amber. Red-colored urine can also occur when blood is present; yellow-brown or greenish-brown urine may be a sign of bilirubin in the urine. Urine clarity refers to how clear the urine is. This could be defined as: clear, slightly cloudy, cloudy, or turbid. "Normal" urine can be clear or cloudy.
A microscopic examination will typically be done when there are abnormal findings on the physical or chemical examination. Cells and other substances that may be seen include the following: red blood cells (RBCs), white blood cells (WBCs), epithelial cells, bacteria, yeast and parasites, trichomonas, casts, and crystals. If the crystals are from substances that are not normally in the urine, they are considered "abnormal." Abnormal crystals may indicate an abnormal metabolic process. Some of these include: calcium carbonate, cystine, tyrosine, and leucine. Urinary presence of some amino acids can be suggestive of amino acid metabolic disorders (chapter 8).
Chemical exam
Much like the CMP, the chemical analysis of a urine sample can be very indicative of biochemical derangement. A review of the following components is helpful in making a clinical diagnosis.
Specific gravity (SG) - Specific gravity is a measure of urine concentration. This test simply indicates how concentrated the urine is.
pH - Urine is typically slightly acidic, about pH 6, but can range from 4.5 to 8. The kidneys play an important role in maintaining the acid–base balance of the body. Therefore, any condition that produces acids or bases in the body, such as acidosis or alkalosis, or the ingestion of acidic or basic foods, can directly affect urine pH.
Protein - The protein test provides an estimate of the amount of albumin in the urine. Normally, there should be no protein (or a small amount of protein) in the urine. When urine protein is elevated, a person has a condition called proteinuria; this could be caused by a variety of health conditions. Healthy people can have temporary or persistent proteinuria due to stress, exercise, fever, aspirin therapy, or exposure to cold, for example.
Glucose - Glucose is normally not present in urine. When glucose is present, the condition is called glucosuria. This condition can result from either an excessively high glucose level in the blood, such as may be seen in individuals with uncontrolled diabetes. Other reducing sugars, galactose or fructose, may also be present in the urine if a metabolic deficiency occurs (section 9.1).
Some other conditions that can cause glucosuria include hormonal disorders, liver disease, medications, and pregnancy. When glucosuria occurs, other tests such as a fasting blood glucose test are usually performed to further identify the specific cause.
Ketones - Ketones are also not normally found in the urine. They are intermediate products of fat metabolism and can be produced when an individual does not eat enough carbohydrates such as in fasting conditions or high-protein diets. When carbohydrates are not available, the body metabolizes fat to generate ATP for baseline metabolic function. Strenuous exercise, exposure to cold, frequent, prolonged vomiting, and several digestive system diseases can also increase fat metabolism, resulting in ketonuria (section 5.2).
In a person who has diabetes, ketones in urine may be an early indication of insufficient insulin. Insufficient insulin response can result in impaired glucose oxidation and consequently results in aberrant fat metabolism. Oxidation of fatty acids provides substrate for ketogenesis, which can cause ketosis and potentially progress to ketoacidosis, a form of metabolic acidosis. Excess ketones and glucose are dumped into the urine by the kidneys in an effort to flush them from the body.
Hemoglobin and myoglobin - The presence of hemoglobin in urine indicates blood in the urine (known as hematuria).
A small number of RBCs are normally present in urine, however, as these numbers elevate, this will result in a positive test result. These results are interpreted with the microscopic exam. For example, a positive test result here with no visible RBCs in the urine would suggest the presence of myoglobin only, which could be due to strenuous exercise or muscle damage.
Leukocyte esterase - Leukocyte esterase is an enzyme present in most white blood cells (WBCs). A few white blood cells are normally present in urine, however, when the number of WBCs in urine increases significantly, this screening test will become positive. When this test is positive and/or the WBC count in urine is high, it may indicate that there is inflammation in the urinary tract or kidneys.
Nitrite - Many normal bacteria can convert nitrate (normally present in urine) to nitrite (not normally present in urine). When bacteria are present in the urinary tract, they can cause a urinary tract infection, which could be diagnosed by a positive nitrite test result.
Bilirubin - Bilirubin is not present in the urine of healthy individuals (figure 2.1). The presence of bilirubin in urine is an early indicator of liver disease and can occur before clinical symptoms such as jaundice develop. Only conjugated bilirubin is present in the urine.
Urobilinogen - Urobilinogen is normally present in urine in low concentrations. It is formed in the intestine from bilirubin, and a portion of it is absorbed back into the blood. Positive test results may indicate liver diseases such as viral hepatitis, cirrhosis, liver damage due to drugs or toxic substances, or conditions associated with increased RBC destruction (hemolytic anemia).
2.1 References and resources
Text
Ferrier, D. R., ed. Lippincott Illustrated Reviews: Biochemistry, 7th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2017, Chapter 27: Nutrition: Overview, Chapter 28: Micronutrients: Vitamins, Chapter 29: Micronutrients: Minerals.
Le, T., and V. Bhushan. First Aid for the USMLE Step 1, 29th ed. New York: McGraw Hill Education, 2018, 65–71.
Figures
Grey, Kindred, Figure 2.1 Heme degradation. 2021. https://archive.org/details/2.2_20210924. CC BY 4.0.
Grey, Kindred, Figure 2.2 Reaction catalyzed by lactate dehydrogenase. 2021. https://archive.org/details/2.4_20210924. CC BY 4.0.
2.2 Vitamins as Coenzymes
Nutritional basics
Many of the metabolic enzymes discussed in this course require essential coenzymes for optimal activity. An individual's nutritional status has the potential to greatly influence their ability to efficiently oxidize fuels, and this can lead to deviations from clinical norms or illness, which would be illustrated on an individual's CMP.
It is important to be aware of the presentation of these nutritional deficiencies as they can manifest as hypoglycemia, different types of anemia, or physiological symptoms.
Overview
Vitamins are organic compounds that, for the most part, we cannot synthesize through endogenous metabolism in adequate quantities (with the exceptions of vitamins B3, D, and K). To address these nutritional needs, we must consume vitamins as part of a balanced diet or supplement through a variety of mechanisms. Below are some key aspects of the roles vitamins play within metabolism and common symptoms associated with deficiencies (table 2.3).
Note
Water-soluble vitamins
- Water-soluble vitamins include: ascorbic acid (vitamin C), thiamin (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), pantothenic acid (vitamin B5), pyridoxine, pyridoxal, and pyridoxamine (vitamin B6), biotin (vitamin B7), and cobalamin (vitamin B12).
- Readily excreted in the urine, toxicity is rare.
- Deficiencies can occur quickly.
- Water-soluble vitamins are precursors of coenzymes for the enzymes of intermediary metabolism.
Fat-soluble vitamins
- Fat-soluble vitamins include: vitamins A, D, K, and E.
- They are released, absorbed, and transported (in chylomicrons) with dietary fat.
- They are not readily excreted, and significant quantities are stored in the liver and adipose tissue.
- Most function as transcriptional regulators.
- Only one fat-soluble vitamin (vitamin K) has a coenzyme function.
- Consumption of vitamins A and D in excess of the dietary reference intakes can lead to accumulation of toxic quantities of these compounds.
Folic acid
Folic acid deficiency is a relatively common vitamin deficiency in the United States, presenting routinely as macrocytic anemia.
- Tetrahydrofolate (THF), the reduced coenzyme form of folate, receives one-carbon fragments from amino acid donors such as serine, glycine, and histidine, and transfers them to intermediates in the synthesis of amino acids, purines, and thymidine monophosphate (TMP, a pyrimidine nucleotide found in DNA).
- Inadequate serum levels of folate can be caused by increased demand (such as the case in pregnancy and lactation), inadequate dietary intake, poor absorption (caused by pathology of the small intestine), alcoholism, or treatment with drugs (for example, methotrexate).
- Folic acid supplementation before conception and during the first trimester has been shown to significantly reduce neural tube defects.
Cobalamin (vitamin B12)
Vitamin B12 is required in humans for two essential enzymatic reactions.
- One of the reactions is the remethylation of homocysteine to methionine, and the other involves the isomerization of methylmalonyl coenzyme A (CoA), which is produced during the degradation of some amino acids (isoleucine, valine, threonine, and methionine) and odd-chain fatty acids (FAs).
- Folic acid (as N5-methyl THF) is also required for one of the reactions needed for remethylation of homocysteine.
- Deficiency of B12 or folate results in elevated Hcy levels (chapter 8), however, only a deficiency of B12 will result in elevated levels of methylmalonic acid.
- Pernicious anemia is a type of vitamin B12 anemia caused by a lack of intrinsic factor. Intrinsic factor (IF) is released from the parietal cells and binds vitamin B12 so that it can be absorbed in the intestines.
- B12 is also important in the synthesis of S-adenosylmethionine (SAM), which plays an integral role in cellular methylation reactions and neurotransmitter synthesis.
Ascorbic acid (vitamin C)
The active form of vitamin C is ascorbic acid.
- Vitamin C is used as a coenzyme in hydroxylation reactions, such as in the hydroxylation of prolyl and lysyl residues of collagen.
- It is required for the maintenance of normal connective tissue as well as for wound healing.
- Vitamin C assists in the absorption of dietary iron by reducing ferric iron to the ferrous form.
Pyridoxine (vitamin B6)
Vitamin B6 is a term that encompasses all derivatives of pyridine including: pyridoxine, pyridoxal, and pyridoxamine.
- Pyridoxine serves as a precursor of the biologically active coenzyme, pyridoxal phosphate (PLP).
- PLP functions as a coenzyme for activation transfer reactions, particularly those that catalyze reactions involving amino acids (section 5.3).
- Isoniazid, a drug commonly used to treat tuberculosis, can induce a vitamin B6 deficiency by forming an inactive derivative with PLP.
- Pyridoxine is the only water-soluble vitamin with significant toxicity. Sensory neuropathies can occur at intakes exceeding five times the Tolerable Upper Limit (UL). This is defined as the maximum amount of daily vitamins and minerals that you can safely take without risk of an overdose or serious side effects.
Thiamine (vitamin B1)
Thiamine pyrophosphate (TPP) is the biologically active form of thiamine and is generated by the transfer of a pyrophosphate group from adenosine triphosphate (ATP) to thiamine.
- TPP is a coenzyme in the formation or degradation of α-ketols by transketolase (section 7.1) and in the oxidative decarboxylation of α-keto acids.
- The activity of both the pyruvate dehydrogenase complex and α-ketoglutarate dehydrogenase can be impaired if thiamine is deficient. This can lead to impaired production of ATP, impaired cellular function, and lactic acidosis.
- TPP is also required by branched-chain α-keto acid dehydrogenase of muscle.
- Activity of erythrocyte transketolase is used to diagnosis a thiamine deficiency.
- Beriberi is a severe thiamine-deficiency syndrome found in geographic areas with poor and restricted diets.
- Wernicke-Korsakoff syndrome can present in individuals with alcohol abuse disorder. Common symptoms include confusion, ataxia, and nystagmus.
Niacin (vitamin B3)
Niacin, or nicotinic acid, is a substituted pyridine derivative. The biologically active coenzyme forms are nicotinamide adenine dinucleotide (NAD+) and its phosphorylated derivative, nicotinamide adenine dinucleotide phosphate (NADP+).
- Nicotinamide is readily deaminated in the body and, therefore, is nutritionally equivalent to nicotinic acid.
- NAD+ and NADP+ serve as coenzymes in oxidation-reduction reactions in which the coenzyme undergoes reduction of the pyridine ring by accepting a hydride ion.
- A deficiency of niacin causes pellagra, which encompasses the three Ds: dermatitis, diarrhea, and dementia.
- Hartnup disorder, characterized by defective absorption of tryptophan, can result in pellagra-like symptoms.
Riboflavin (vitamin B2)
The two biologically active forms of B2 are flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), formed by the transfer of an adenosine monophosphate moiety from ATP to FMN.
- FMN and FAD are each capable of reversibly accepting two hydrogen atoms, forming FMNH2 or FADH2. FMN and FAD are bound tightly, or covalently, to flavoenzymes that catalyze the oxidation or reduction of a substrate.
- Riboflavin deficiency is not associated with a major human disease, although it frequently accompanies other vitamin deficiencies.
Biotin (vitamin B7)
Biotin is a coenzyme in carboxylation reactions, in which it serves as a carrier of activated carbon dioxide (coenzyme for acetylCoA carboxylase and pyruvate carboxylase).
- Biotin is covalently bound to the ε-amino group of lysine residues in biotin-dependent enzymes.
- Biotin deficiency does not occur naturally because the vitamin is widely distributed in food.
- Excessive consumption of raw egg white as a source of protein can cause symptoms of biotin deficiency. Symptoms may include: dermatitis, glossitis, loss of appetite, and nausea. Raw egg white contains avidin, which is a glycoprotein that tightly binds biotin and prevents its absorption from the intestine.
Pantothenic acid
Pantothenic acid is a component of CoA, which functions in the transfer of acyl groups.
- CoA contains a thiol group that carries acyl compounds as activated thiol esters. Examples of such structures are succinyl-CoA, fatty acyl-CoA, and acetyl-CoA.
- Pantothenic acid is also a component of the acyl carrier protein domain of fatty acid synthase (section 4.4).
- The vitamin is widely distributed in a variety of foods, and deficiency is not well characterized in humans.
Vitamin A
The retinoids are a family of molecules that are related to dietary retinol (vitamin A).
- Vitamin A (and its metabolites) are important for vision, reproduction, growth, immune function, and maintenance of epithelial tissues.
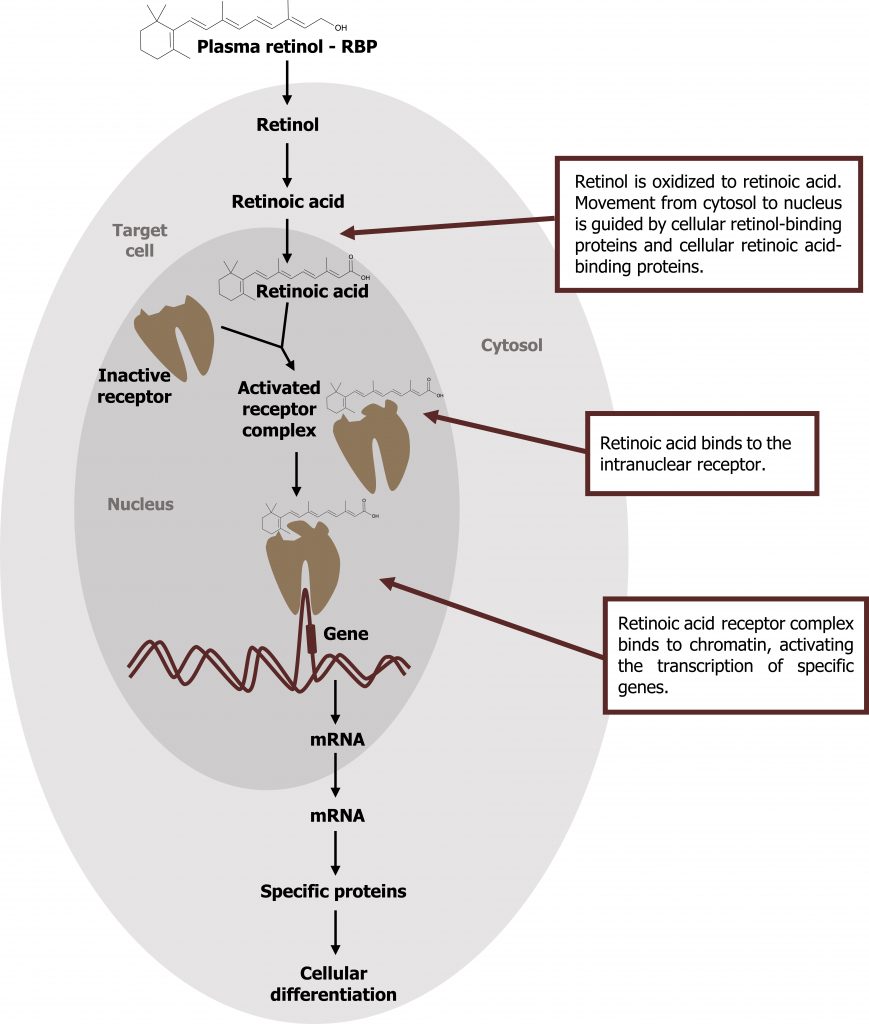
- Retinoic acid is derived from the oxidation of retinol and mediates most of the actions of the retinoids.
- Retinol is oxidized to retinoic acid. Retinoic acid binds specifically to a family of nuclear receptors (retinoic acid receptors, RAR) and modulates gene expression in target tissues, such as epithelial cells. The activated retinoic acid‒RAR complex binds to response elements on DNA and recruits activators or repressors to regulate retinoid-specific mRNA synthesis (figure 2.3).
Vitamin D
The D vitamins are a group of sterols that have a hormone-like function.
- The active molecule, 1,25-dihydroxycholecalciferol (calcitriol), binds to intracellular receptor proteins. The receptor complex interacts with DNA in the nucleus of target cells in a manner similar to that of vitamin A and either selectively stimulates or represses gene transcription.
- The most prominent actions of calcitriol are to regulate the plasma levels of calcium and phosphorus. Within the gastrointestinal tract, calcitriol increases the transcription of calcium transport proteins, calbindin-D proteins, which results in increased uptake of calcium. It also increases reabsorption of phosphorus through a similar mechanism.
Vitamin K
- The primary role of vitamin K is to serve as a coenzyme in the carboxylation of glutamic acid residues; this post-translational modification is required for the functioning of many proteins required for blood clotting.
- Vitamin K is required in the hepatic synthesis of prothrombin (factor II) and factors VII, IX, and X (figure 2.4).
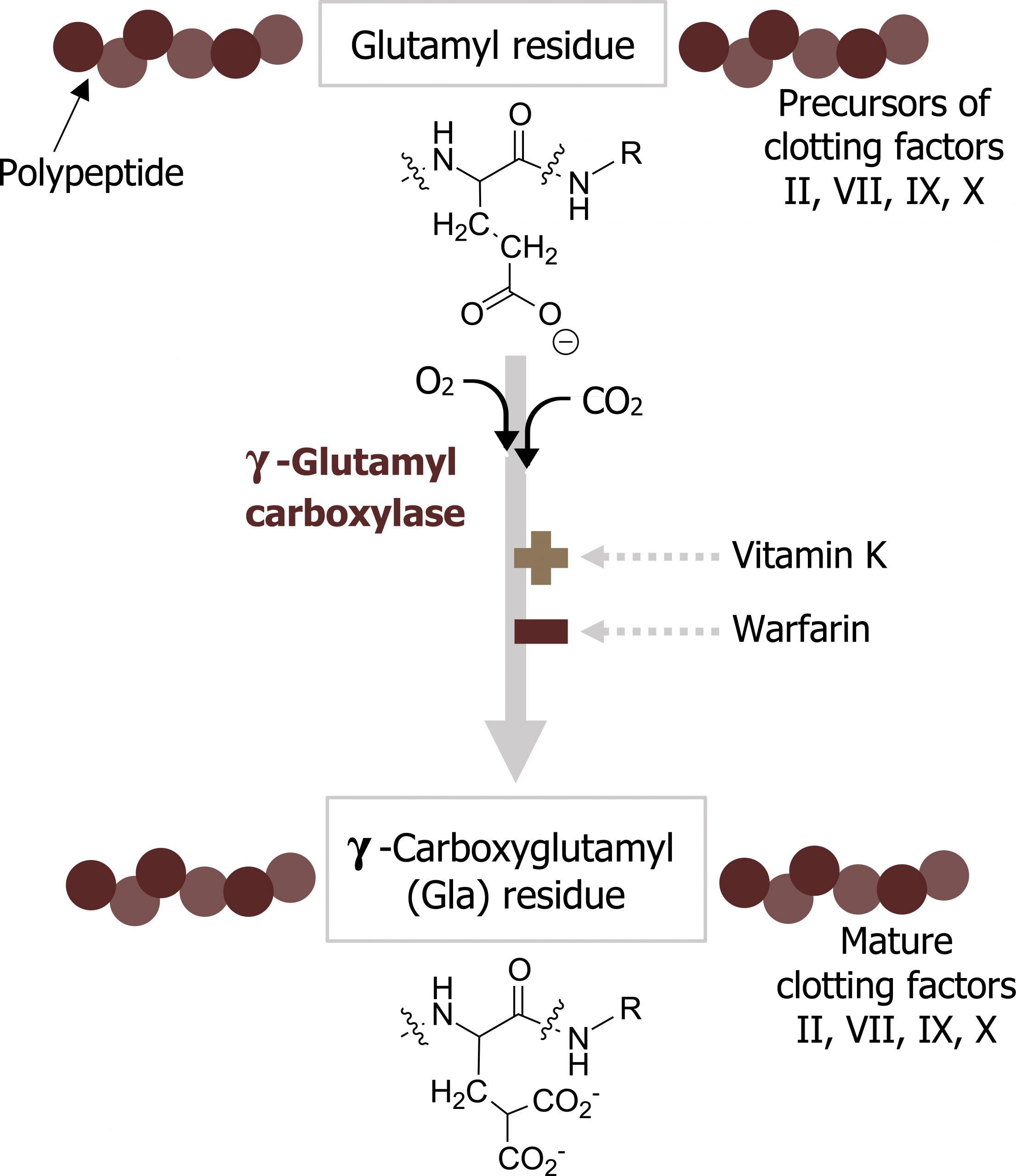
- The formation of carboxy-glutamyl (Gla) residues is sensitive to inhibition by warfarin, an analog of vitamin K that inhibits vitamin K epoxide reductase (VKOR), the enzyme required to regenerate the functional hydroquinone form of vitamin K.
Vitamin E
The E vitamins consist of eight naturally occurring tocopherols, of which α-tocopherol is the most active.
- The primary function of vitamin E is as an antioxidant in prevention of the nonenzymatic oxidation of cell components.
- Vitamin E deficiency in adults is usually associated with defective lipid absorption or transport.
| Vitamin | Other names | Active form | Function | Deficiency | Signs and symptoms | Toxicity | Notes |
|---|---|---|---|---|---|---|---|
| Water soluble | |||||||
| Vitamin B9 | Folic acid | Tetrahydrofolic acid | Transfer one-carbon units; synthesis of methionine, purines, and thymidine monophosphate | Megaloblastic anemia Neural tube defects |
Anemia Birth defects |
None | Administration of high levels of folate can mask vitamin B12 deficiency |
| Vitamin B12 | Cobalamin | Methylcobalamin Deoxyadenosyl cobalamin |
Coenzyme for reactions: • homocysteine → methionine • methylmalonyl-CoA → succinyl-CoA |
Pernicious anemia Dementia Spinal degeneration |
Megaloblastic anemia Neuropsychiatric symptoms |
None | Pernicious anemia is treated with intramuscular or high-dose oral vitamin B12 |
| Vitamin C | Ascorbic acid | Ascorbic acid | Antioxidant Coenzyme for hydroxylation reactions, for example: In procollagen: • proline → hydroxyproline • lysine → hydroxylysine |
Scurvy | Sore, spongy gums Loose teeth Poor wound healing |
None | Benefits of supplementation not established in controlled trials |
| Vitamin B6 | Pridoxine Pyridoxamine Pyridoxal |
Pyridoxal phosphate |
Coenzyme for enzymes, particularly in amino acid metabolism | Rare | Glossitis Neuropathy |
Yes | Deficiency can be introduced by isoniazid Sensory neuropathy occurs at high doses |
| Vitamin B1 | Thiamine | Thiamine pyrophosphate | Coenzyme of enzymes catalyzing: • pyruvate → acetyl-CoA • α-ketoglutarate → succinyl-CoA • ribose 5-P + xylulose 5-P → sedoheptulose 7-P + glyceraldehyde 3-P • branched-chain α-keto acid oxidation |
Beriberi Wernicke- Korsakoff syndrome (most common in alcoholics) |
Tachycardia, vomiting, convulsions Apathy, loss of memory, dysregulated eye movements |
None | |
| Niacin | Nicotinic acid Nicotinamide |
NAD+ NADP+ |
Electron transfer | Pellagra | Dermatitis Diarrhea Dementia |
None | High doses of niacin used to treat hyperlipidemia |
| Vitamin B2 | Riboflavin | FMN FAD |
Electron transfer | Rare | Dermatitis Angular stomatitis |
None | |
| Biotin | Enzyme-bound biotin | Carboxylation reactions | Rare | None | Consumption of large amounts of raw egg whites (which contain a protein, avidin, that binds biotin) can induce a biotin deficiency | ||
| Pantothenic acid | Coenzyme A | Acyl carrier | Rare | None | |||
| Fat soluble | |||||||
| Vitamin A | Retinol Retinal Retinoic acid β-Carotene |
Retinol Retinal Retinoic acid |
Maintenance of reproduction Vision Promotion of growth Differentiation and maintenance of epithelial tissues Gene expression |
Infertility Night blindness Retardation of growth Xerophthalmia |
Increased visual threshold Dryness of cornea |
Yes | Β-Carotene not acutely toxic, but supplementation is not recommended Excess vitamin A can increase incidence of fractures |
| Vitamin D | Cholecalciferol Ergocalciferol |
1,25-dihydroxy- cholecalciferol |
Calcium uptake Gene expression |
Rickets (in children) Osteomalacia (in adults) |
Soft, pliable bones | Yes | Vitamin D is not a true vitamin because it can be synthesized in skin; application of sunscreen lotions or presence of dark skin color decreases this synthesis |
| Vitamin K | Menadione Menaquinone Phylloquinone |
Menadione Menaquinone Phylloquinone |
γ-Carboxylation of glutamate residues in clotting and other proteins | Newborn Rare in adults |
Bleeding | Rare | Vitamin K produced by intestinal bacteria Vitamin K deficiency common in newborns Intramuscular treatment with vitamin K is recommended at birth |
| Vitamin E | α-Tocopherol | Any of several tocopherol derivatives | Antioxidant | Rare | Red blood cell fragility leads to hemolytic anemia | None | Benefits of supplementation not established in controlled trials |
Table 2.3: Summary table of vitamins.
2.2 References and resources
Text
Ferrier, D. R., ed. Lippincott Illustrated Reviews: Biochemistry, 7th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2017, Chapter 27: Nutrition: Overview, Chapter 28: Micronutrients: Vitamins, Chapter 29: Micronutrients: Minerals.
Le, T., and V. Bhushan. First Aid for the USMLE Step 1, 29th ed. New York: McGraw Hill Education, 2018, 65–71.
Figures
Ferrier D. Figure 2.3 Mechanism of action of Vitamin A. Adapted under Fair Use from Figure 28.20 Action of the retinoids. Lippincott Illustrated Reviews Biochemistry. 7th Ed. pp388. 2017. Chemical structure by Henry Jakubowski.
Grey, Kindred, Figure 2.4 Vitamin K stimulates the maturation of clotting factors. 2021. Chemical structure by Henry Jakubowski. https://archive.org/details/2.6_20210924. CC BY 4.0.
Tables
Table 2.3 adapted from Ferrier, D. R., ed. Lippincott Illustrated Reviews: Biochemistry, 7th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2017.

