19 Extracellular Matrix
Learning Objectives
- Define the general structure and function of the glycocalyx and extracellular matrix.
- Describe the function and structure of basement membranes (basal lamina).
- Describe the membrane proteins involved in the adhesion of cells to noncellular surfaces.
- Compare and contrast the structures and functions of the different cell junctions.
- Describe the membrane proteins involved in cell–cell adhesion.
About this Chapter
Most animal cells release materials into the extracellular space. The primary components of these materials are glycoproteins and the protein collagen. Collectively, these materials are called the extracellular matrix. Not only does the extracellular matrix hold the cells together to form a tissue, but it also allows the cells within the tissue to communicate with each other.
19.1 Extracellular Matrix
Extracellular matrix
The extracellular matrix consists of a network of substances secreted by cells (figure 19.1). This dynamic structure has many roles in cellular adhesion, signaling, and overall cellular organization.
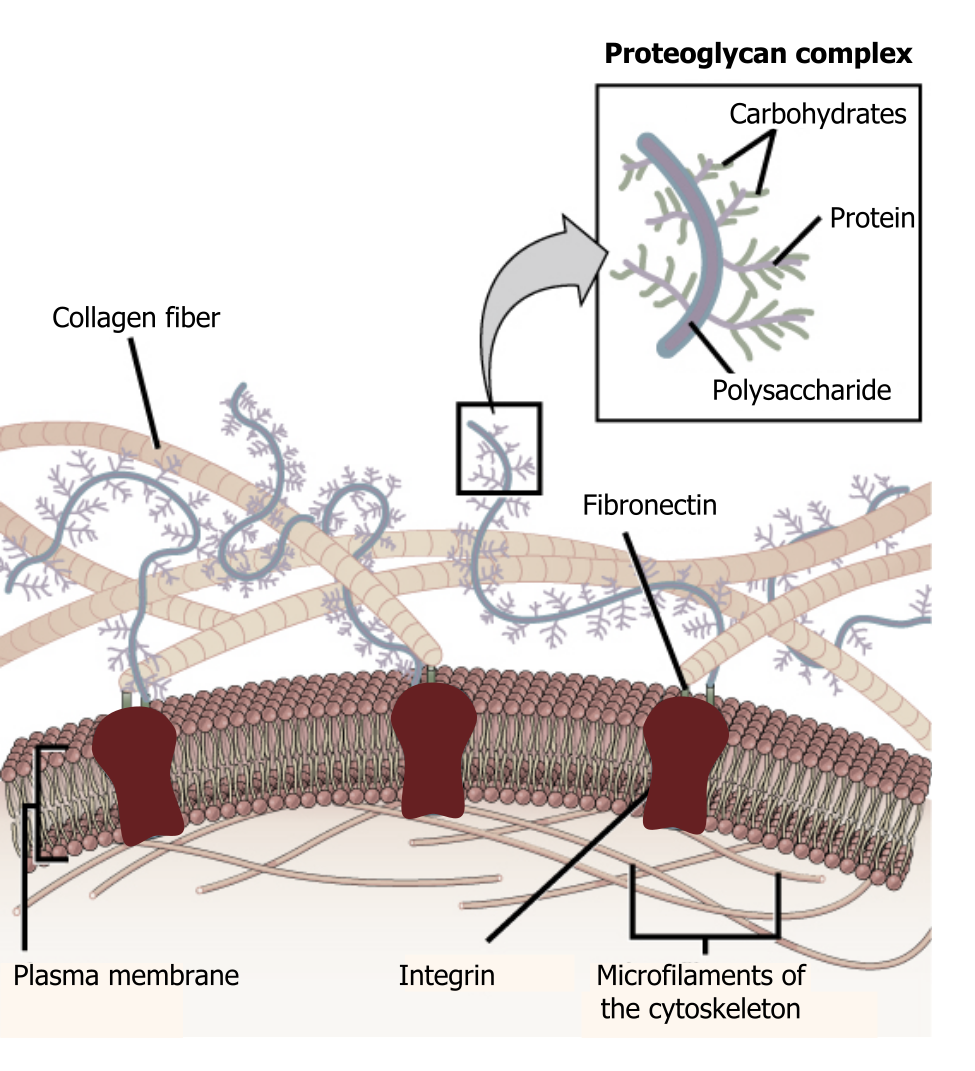
Glycocalyx
In addition to the many proteins in the matrix, there are some essential carbohydrate components that make up the glycocalyx. This is a carbohydrate “coat” that forms a barrier around the plasma membrane, can bind regulatory factors, and can mediate cell–cell interactions.
Proteins of the matrix
The major components of the extracellular matrix form a fibrous, secreted network.
Fibronectin
Fibronectin is a dimer held together by disulfide bonds. These dimers assemble in structural modules that serve as binding sites for other proteins such as heparin, collagen, and fibrin. These proteins are the “matrix” that allows other proteins to dock, and it has RGD loops that serve as integrin binding sites.
Collagen
Collagen is synthesized by fibroblasts and is the most abundant protein in the body. There are twenty-seven types of collagen, and it is often a triple helix, stabilized by hydrogen bonding. There are additional nonfibrillar forms that can form web-like structures. Synthesis of collagen requires vitamin C for formation of hydroxyproline.
As collagen is a major component to connective tissue, it plays key roles in tendons, cornea, blood vessels, hair, and cartilage. Disorders of collagen synthesis are implicated in osteogenesis imperfecta, dwarfism, and skeletal deformities.
Proteoglycans
Structurally proteoglycans contain both proteins plus glycosaminoglycans (carbohydrates) and take on a bottle-brush structure (figure 19.1). They assemble into larger complexes, bound to a central hyaluronic acid. The overall structure is negatively charged, which attracts water, allowing it to function as a cushion.
Laminin
Laminin consists of three glycoproteins linked through disulfide bonds. They bind cell-surface receptors and are important during development and cell migration.
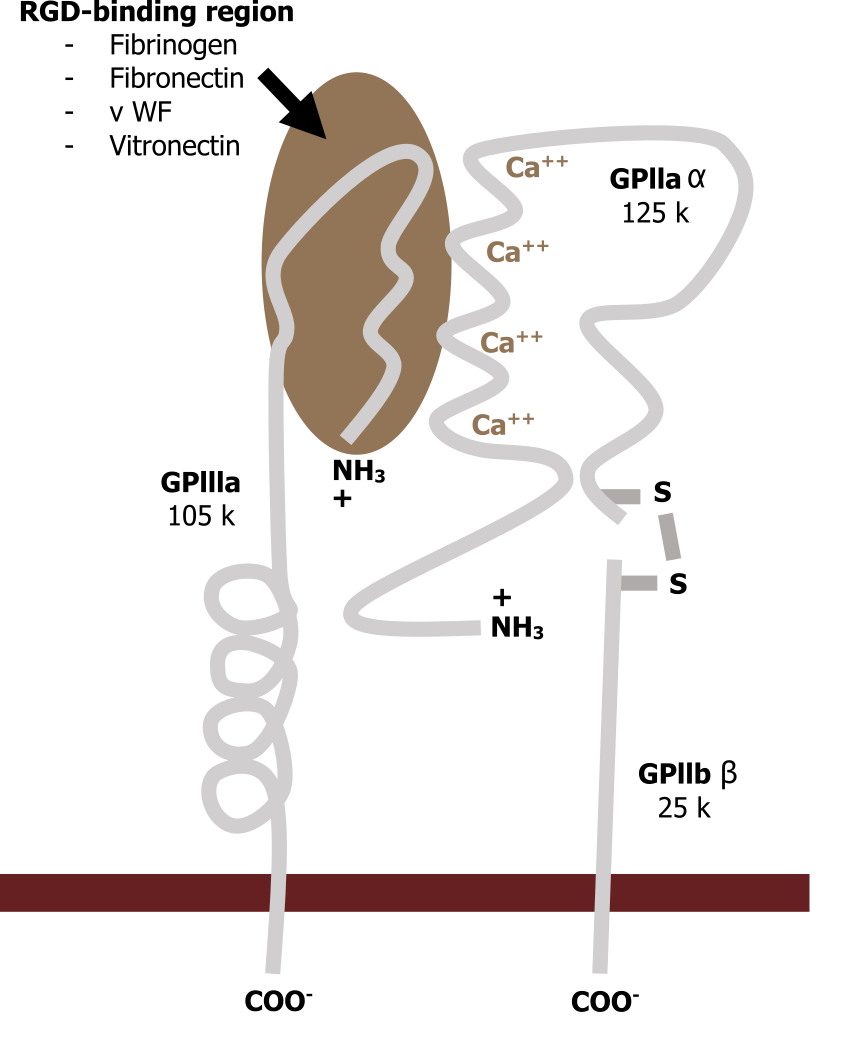
Integrins
Integrins span the plasma membrane and connect the matrix to the cellular environment (inside-out or outside-in signaling). They are associated with fibronectin (RGD sequences) on the extracellular side and have both active and inactive states (figure 19.2).
Matrix metalloproteinases
The matrix is a dynamic component and is remodeled as a part of normal growth and development. It can also be reorganized as a result of disease or pathology such as arthritis or atherosclerosis.
A family of enzymes, termed matrix metalloproteinases, is responsible for this remodeling, and they require metal for catalysis. Zinc is the typical cofactor, although some enzymes in the family require cobalt.
Cell adhesion to the substratum
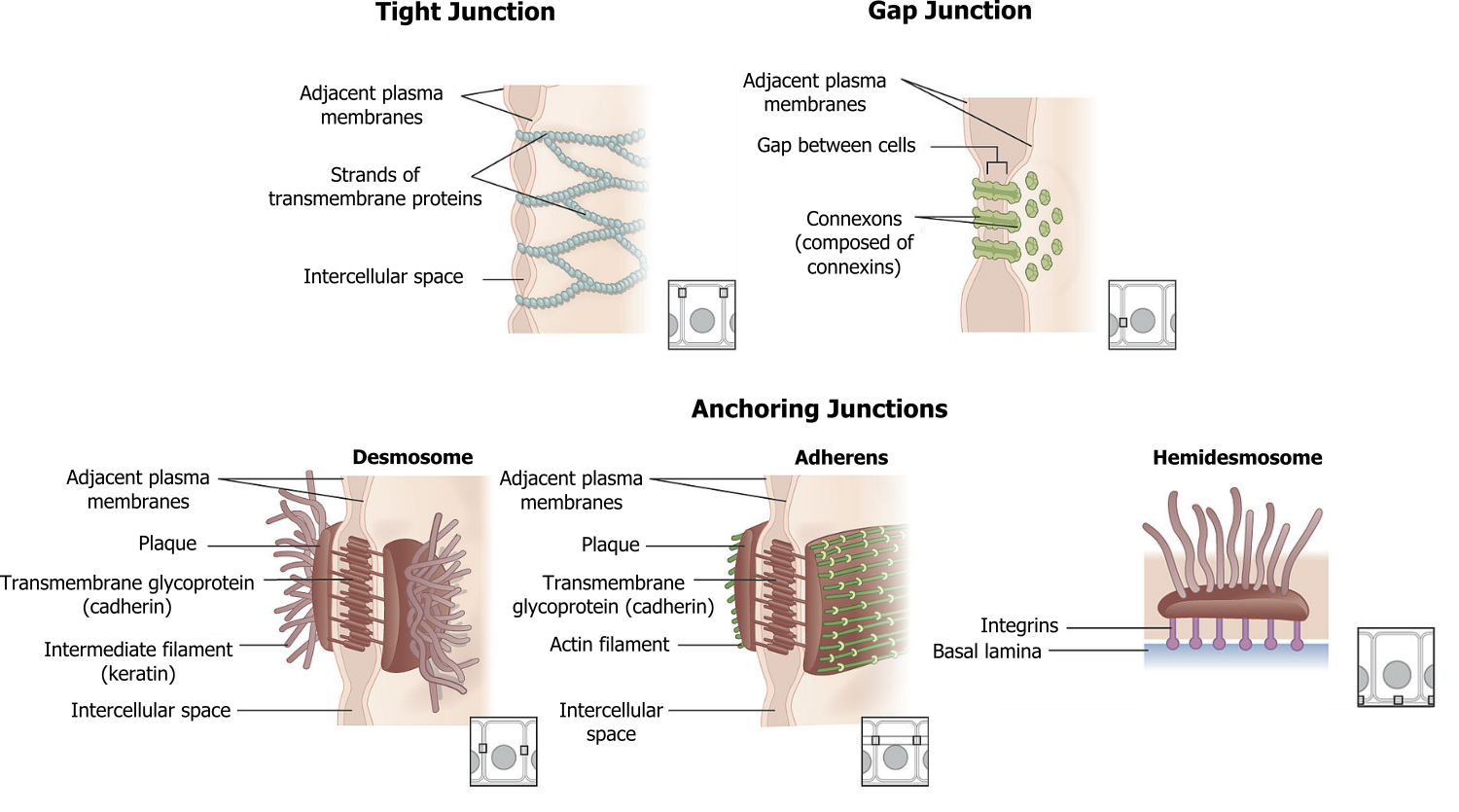
Focal adhesions and hemidesmosomes (figure 19.3) serve to anchor cells to the substratum.
Focal adhesions are a cluster of rapidly assembling and disassembling proteins that involves a cluster of integrins connected to actin in the cytoskeleton.
Hemidesmosomes are the tightest of all in vivo attachments. They consist of intermediate filaments (keratin) and form an attachment between the basal surface of epithelial cells to the basement membrane.
Cell–cell adhesion
Cells attach to one another through interactions of cell adhesion molecules (CAMs). These interactions can be transient involving one protein on one cell and one on another cell. There are several major classes of CAMs with biological relevance. Their roles are summarized in the table below.
| CAM | Characteristics | Role |
|---|---|---|
| Selectins (E,L,P) | Membrane glycoproteins | Bind carbohydrate ligands; transient interactions |
| Ig superfamily | Calcium independent | Modulate interactions with immune cells |
| Integrins | Interacts with the Ig superfamily | RGD domains for interaction with the matrix |
| Cadherins | Calcium-dependent glycoproteins | Join similar cells |
Table 19.1: Major classes of CAMs with biological relevance.
Cell–cell adhesive junctions
There are four kinds of connections between cells:
- Adherens junctions are composed of cadherins and anchor the cell through interactions with actin filaments.
- Tight junctions join adjacent animal cells and function as barriers; they are very important in capillary endothelial cells.
- Desmosomes join two cells together through interactions with keratin.
- Gap junctions act as channels between cells for direct chemical communication.
19.1 References and resources
Text
Clark, M. A. Biology, 2nd ed. Houston, TX: OpenStax College, Rice University, 2018, Chapter 4: Cell Structure, Chapter 5: Structure and Function of the Plasma Membranes.
Karp, G., and J. G. Patton. Cell and Molecular Biology: Concepts and Experiments, 7th ed. Hoboken, NJ: John Wiley, 2013, Chapter 7: Interactions between Cells and Their Environment.
Le, T., and V. Bhushan. First Aid for the USMLE Step 1, 29th ed. New York: McGraw Hill Education, 2018, 50–51.
Figures
Grey, Kindred, Figure 19.1 Overview of the extracellular matrix. 2021. https://archive.org/details/19.1_20210926. CC BY 4.0. Adapted from Figure 16. CC BY 4.0. From Open Oregon.
Grey, Kindred, Figure 19.2 Schematic of integrin structure… 2021. CC BY SA 3.0. Adapted from Integrin by Odysseus1479. CC BY SA 3.0. From Wikimedia Commons.
Grey, Kindred, Figure 19.3 Summary of cell adhesion mechanisms. 2021. https://archive.org/details/19.3_20210926. CC BY 3.0. Adapted from 402 Types of Cell Junctions new by OpenStax College. CC BY 3.0. From Wikimedia Commons.

