Chapter 6: Diagnosing Plant Damage
Chapter Contents
Diagnosis of plant problems is often a very difficult task since there can be many different causes for a given symptom, not all of which are pathogenic organisms. Soil nutrition and texture, weather conditions, lighting, and many other environmental and cultural conditions influence the overall health of a plant. Insect damage can sometimes be confused with plant disease caused by microorganisms or abiotic factors. Knowing a complete history of the plant is essential to making an accurate diagnosis. Also, a plant specimen should be in the early stages of deterioration when it is examined in order for an accurate diagnosis to be made. Once it has decayed, secondary organisms invade the tissue and evidence of the primary pathogen is often obscured.
For these reasons, it is difficult to construct a foolproof key for the diagnosis of plant problems. Even with the necessary laboratory equipment at one’s disposal, it is often difficult to determine the exact cause of a plant’s problem.
The following pages provide an aid to diagnosing some of the common problems of urban plants. This chapter was constructed to help solve consumer’s plant problems — it is not meant for diagnosis of commercial problems or use by laboratory diagnosticians. The information provided is by no means comprehensive, and other resources will be needed for many of your diagnoses.
A Systematic Approach to Diagnosing Plant Damage
Determining what factors caused damage to a plant requires an inquisitive, investigative approach combined with careful observation and the ability to put all the pieces together to reconstruct the event(s) that produced the plant damage. Accurate diagnosis must be made before corrective action can be taken. Even if no corrective measures are available, there is satisfaction in simply knowing what the problem is and what its future development might be.
Living (biotic) factors: Living organisms such as pathogens (fungi, bacteria, viruses, nematodes), and pests (insects, mites, mollusks, rodents, etc.). With living factors, “Something is missing, and something is gained.”
Nonliving (abiotic) factors: Mechanical factors (breakage, abrasions, etc.), physical/environmental factors (extremes of temperature, light, moisture, oxygen, lightning), and chemical factors (chemical phytotoxicities, nutritional disorders, etc.).
If we suspect that it is a living damaging factor, we will look for signs and symptoms to distinguish between pathogens and pests. If the accumulated evidence suggests that it is a pathogen, we will seek evidence to distinguish among fungal, bacterial, viral pathogens, and nematodes. If the evidence indicates the damaging factor is an insect or other animal, we will seek further evidence to distinguish between sucking and chewing types.
The probability of a correct diagnosis based on only one or two clues or symptoms is low. Similarities of symptoms produced on the same plant by completely different factors frequently make the use of symptoms alone inadequate.
In diagnosing plant damage, a series of deductive steps can be followed to gather information and clues from the big, general situation down to the specific, individual plant or plant part. Through this systematic, diagnostic process of deduction and elimination, the most probable cause of the plant damage can be determined. Steps to follow in gathering diagnostic information are presented below. Each step will then be expanded and guidelines presented as we proceed through the diagnostic process. We will first identify the problem, then attempt to distinguish between living and nonliving damaging factors based on the observed damage patterns, development of the patterns with time, and other diagnostic signs.
Factors causing plant damage can be grouped into two major categories: If evidence indicates that the damage is being caused by a nonliving factor, we will seek further evidence as to whether the initial damage is occurring in the root or aerial environment. We will then attempt to determine if the damage results from mechanical factors, from extremes in physical factors (such as extremes of temperature, light, moisture, or oxygen), or from chemical factors (phytotoxic chemicals or nutritional disorders). Once we have identified the plant and limited the range of probable causes of the damage, we can obtain further information to confirm our diagnosis from reference books, specialists such as plant pathologists, entomologists, horticulturists, and/or laboratory analyses.
Model for Diagnosing Plant Damage
I. Define the Problem: Determine that a “real” problem exists.
- Identify plants and know characteristics. Establish what the “normal” plant would look like at this time of year. Describe the “abnormality”: Symptoms & Signs.
- Examine the entire plant and its community. Determine the primary problem and part of the plant where initial damage occurred.
II. Look for Patterns: On more than one plant? On more than one plant species?
- Understand nonuniform damage pattern (scattered damage on one or only a few plant species) is indicative of living factors (pathogens, insects, etc.).
- Understand uniform damage pattern over a large area (i.e., damage pattern on several plant species) and uniform pattern on the individual plant and plant parts indicates nonliving factors (mechanical, physical, or chemical factors).
- Compare patterns of living and nonliving factors on plant community, plant, plant part.
III. Delineate Time-Development of Damage Pattern
- Progressive spread of the damage on a plant onto other plants or over an area with time indicates damage caused by living organisms.
- Damage occurs, does not spread to other plants or parts of the affected plant. There is a clear line of demarcation between damaged and undamaged tissues. These clues indicate nonliving damaging factors.
IV. Determine Causes of the Plant Damage: Ask questions and gather information.
- Distinguish among living factors:
- Symptoms and signs of pathogens
- Symptoms and signs of insects, mites, and other animals
- Distinguish among nonliving factors:
- Mechanical factors
- Physical factors (temperature extremes, light extremes, oxygen and moisture extremes)
- Chemical factors (damage patterns in fields and other plantings, injury patterns on individual plants, pesticide-pollutant phytotoxicities/damage patterns, nutritional disorders)
- References (check reports of damaging factors on identified plant); may need laboratory analysis to narrow range of probable causes.
V. Synthesis of Information to Determine Probable Causes
Define the Problem
Identify Plant and Know Characteristics
Is the growth and appearance of the identified plant normal? Is it abnormal?
Determine that a real problem exists. It is essential that the plant be identified (genus, species, and cultivar or variety) so that the normal appearance of that plant can be established either by personal knowledge or by utilizing plant reference books. Many horticultural plants or structures on those plants such as fruits, seeds, lenticels, etc. may appear to be abnormal to the person who is not familiar with the specific plant. For example, the ‘Sunburst’ honey locust might appear to be suffering from a nutrient deficiency because of its chlorotic yellow-green leaf color, but it was selected because of this genetic characteristic. It is not abnormal for this plant; therefore, it is not a problem.
Always compare the typical diseased plant with a healthy or normal plant, since normal plant parts or seasonal changes are sometimes mistakenly assumed to be evidence of disease. Examples are the brown, spore-producing bodies on the lower surface of leaves of ferns.
These are the normal propagative organs of ferns. Also in this category are the small, brown, club-like tips that develop on arborvitae foliage in early spring. These are the male flowers, not deformed shoots. Small galls on the roots of legumes, such as beans and peas, are most likely nitrogen-fixing nodules essential to normal development and are not symptoms of root-knot nematode infection. The leaves of some plants, such as some rhododendron cultivars, are covered by conspicuous fuzz-like epidermal hairs. This is sometimes thought to be evidence of disease, but it is a normal part of the leaf. Varieties of some plants have variegated foliage that may resemble certain virus diseases. These examples illustrate the importance of knowing what the normal plant looks like before attributing some characteristic to disease.
In describing the plant “abnormality,” distinguish between symptoms and signs. Symptoms are changes in the growth or appearance of the plant in response to living or nonliving damaging factors. Many damaging factors can produce the same symptoms; symptoms are not definitive. Signs are evidence of the damaging factor (pest or pathogen life stages, secretions, mechanical damage, chemical residues, records of weather extremes or chemical applications, damage patterns). Patterns of damage are excellent signs and are definitive diagnostic clues.
Examine the Entire Plant and Its Community
In defining a plant problem, it is essential to determine the real primary problem. There are foliage symptoms that may occur due to root damage. The primary problem would be root damage, not chlorosis of the foliage — examine the roots. In general, if the entire top of the plant or entire branches are exhibiting abnormal characteristics, examine the plant downward to determine the location of the primary damage. Look for the factor causing the damage at the periphery of the plant damage.
Some pathogens and insects as well as nonliving factors are only damaging if the plant has been predisposed by other primary factors. For example, borers generally only attack trees that are already predisposed by moisture or other physical stress. Premature dropping of leaves by foliage plants (like Ficus benjamina) and of needles by conifers frequently causes alarm. Evergreen plants normally retain their leaves for 3-6 years and lose the oldest gradually during each growing season. This normal leaf drop is not noticed. However, prolonged drought or other stress factors may cause the tree as a whole to take on a yellow color for a short period and may accelerate leaf loss. If the factors involved are not understood, this often causes alarm. The leaves that drop or turn yellow are actually the oldest leaves on the tree, and their dropping is a protective mechanism which results in reduced water loss from the plant as a whole.
Normal versus abnormal needle drop or leaf drop from evergreens

Non-deciduous plants normally retain their leaves for several years but eventually they fall. This drop is usually gradual, and production of new leaves obscures the loss of older leaves.
Normal: If drop is confined to older leaves, alarm is unnecessary because it is a normal response to a condition of stress (e.g., drought). Unfavorable growing conditions such as drought may accelerate leaf fall so that it becomes apparent and of concern.
Abnormal: If newly produced leaves are lost, it is a problem. The drop of current year’s leaves may result from a pathogen or insect attack or from chemical deficiencies or toxicities.
Look for Patterns
Here is where we start making the distinction between living and nonliving factors that cause plant damage.
Understand Nonuniform Damage Pattern
Living factors
There is usually no discernible, widespread pattern of damage on a planting. Damage produced by living organisms, such as pathogens or pests, generally results from their using the plant as a food source. Living organisms are generally rather specific in their feeding habits and do not initially produce a wide-spread, discernible damage pattern. Plants become abnormal. Tissues are destroyed or removed, become deformed, or proliferate into galls.
Living organisms are specific (damage may be greatest on or limited to one species of plant).
Living organisms multiply and grow with time; therefore, they rarely afflict 100% of the host plants at one time. The damage is progressive over time. Likewise, the damage generally is initially limited to only one part of the plant and spreads from that initial point of attack with time.
Living organisms usually leave “signs” like excrement, cast skins, mycelium, eggs, etc.
Understand Uniform Damage Pattern
Nonliving factors
Damage patterns produced by nonliving factors such as frost or applications of toxic chemicals are generally recognizable and widespread. Damage will appear on all leaves of a certain age (for example, on all the leaves forming the plant canopy at the time a toxic spray was applied) or exposure (all leaves not shaded by overlapping leaves on the southwest side of a plant may be damaged by high temperatures resulting from intense sunlight). Damage will likely appear on more than one type or species of plant (look for similar damage patterns on weeds, neighboring plants, etc.) and over a relatively large area.
Compare Patterns
The following figures provide a comparison of patterns of living and nonliving factors on plant community, plant, and plant part and discuss these factors.
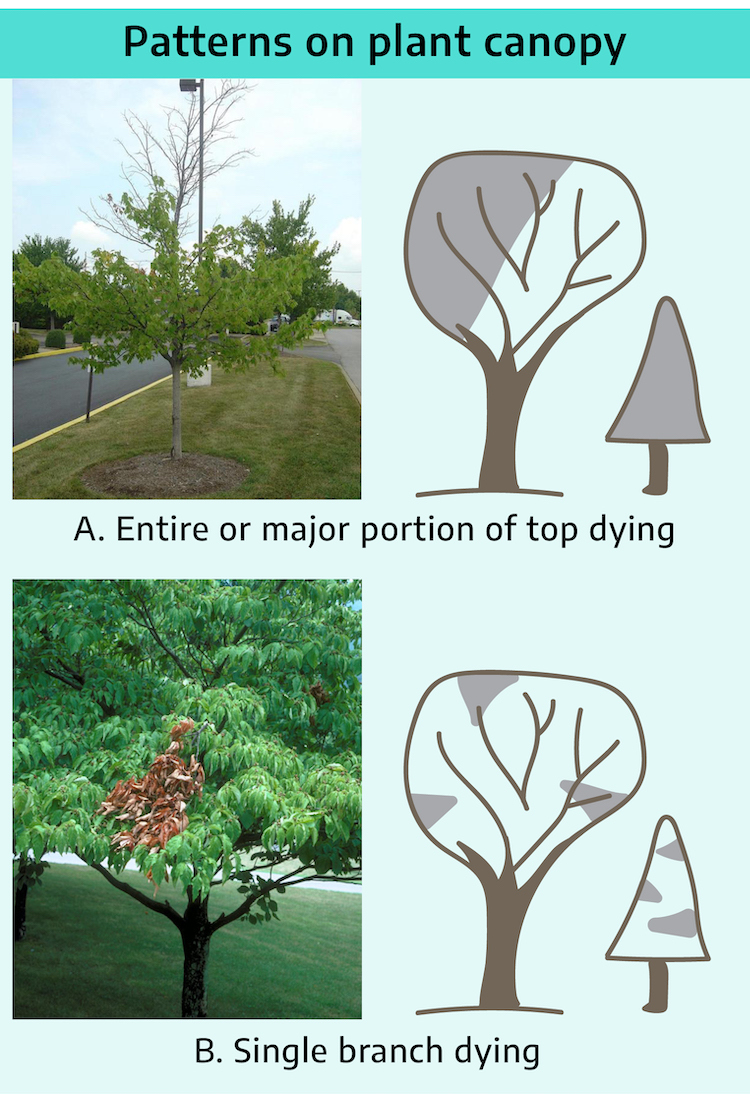
A. Entire or major portion of top dying: If all or a major portion of a tree or shrub dies, suspect a problem with the roots. Look for damaging factors at junction of normal and abnormal plant tissue.
Gradual decline of entire plant or a major portion of it is caused by living factors such as Armillaria root rot, Verticillium wilt, and root weevil.
Sudden decline is generally caused by a nonliving factor such as a toxic chemical in the soil or drastic climatic changes such as freezing or drought.
B. Single branch dying: If scattered damage occurs in the plant canopy, suspect that the primary problem is related to the foliage or aerial environment, not the roots.
Gradual death of a branch: If scattered branches start to decline and eventually die, suspect a living organism such as a canker pathogen, a shoot blight, or borers.
Sudden death of a branch: If a branch dies suddenly, and especially if affected branches are concentrated on one side of the plant, suspect a nonliving factor such as weather (wind, snow, etc.), animal damage, or chemical drift.
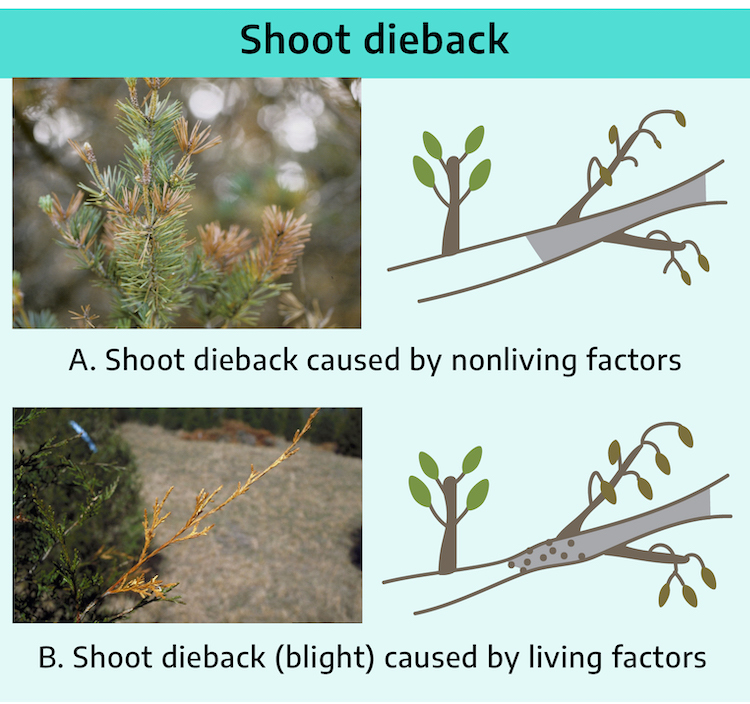
A. Shoot dieback caused by nonliving factors: Sudden dying back of a shoot usually indicates a nonliving cause such as climatic or chemical damage, not a living factor. Damage caused by nonliving factors usually results in a sharp line between affected and healthy bark.
If dieback is more gradual and there is also cracking of the bark and wood, suspect winter injury.
B. Shoot dieback (blight) caused by living factors: Gradual decline of shoots and retention of dead leaves may indicate a living factor.
The margin between affected and healthy tissue is often irregular and sunken.
There may be small, pin-like projections or bumps over the surface of dead bark. These are spore-producing structures of pathogenic fungi.
However, small, woody bumps radiating from all sides of twigs of dwarf Alberta spruce are pulvinus, woody projections where needles were attached. This is a taxonomic identifying characteristic of spruce.

Death of the tips of conifer needles producing a uniform pattern usually indicates a nonliving factor such as a toxic chemical or unfavorable climatic condition. Air pollutants frequently cause tip burn on conifers as do certain soil-applied herbicides or excess fertilizer.
Drought and freezing may have a similar effect. In these cases all needles of a specific growth period are usually affected, and usually the same length on each needle is affected.
The margin between the affected tissue (usually reddish brown) and healthy tissue is sharp and distinct.
Damage by living organisms, such as fungi and insects, to needles usually occurs in a random, scattered pattern and rarely kills all needles of a particular growth period. Needles are usually affected over varying lengths and often appear straw yellow or light tan in color. Black fruiting bodies of causal fungus may be present on diseased needles.
Delineate Development
As already mentioned, another clue for distinguishing between living and nonliving factors causing plant damage is to observe the development of the pattern.
Living organisms generally multiply with time and produce an increasing spread of the damage over a plant or planting with time, and therefore are progressive.
Spots are usually uniformly and evenly distributed over the leaf surface, and generally will be of uniform size. Color is usually uniform across the spot.
Injury from chemicals taken up by plants from soil through roots or from air through leaves usually results in scorching (necrosis) of leaf margins and interveinal areas. If severe, necrotic tissue may drop out giving a ragged appearance. Similar patterns are produced by moisture stress. If uptake of toxic chemical is to a fully expanded leaf, toxicity is marginal and interveinal. If uptake is to an unexpanded leaf, toxicity occurs in veins.
Nonliving factors generally damage the plant at a given point in time; for example, death of leaf tissue caused by a phytotoxic chemical is immediate and does not spread with time. There are exceptions. If a nonliving damaging factor is maintained over time, the damage will also continue to intensify with time. For example, if a toxic soil or air chemical is not removed, damage to plants within the contaminated area will continue to develop, but damage will not spread to plants in uncontaminated areas. Nonliving factors are not progressive. This again reemphasizes the necessity of piecing together multiple clues to identify the most probable factor causing plant damage.
Determine Causes
Patterns of damage distribution and time patterns in development of damage have been valuable in making the gross distinction between damage caused by living factors and damage caused by nonliving factors. Additional clues must be obtained to distinguish among factors within the living and nonliving categories.
Distinguish Among Living Factors
To further identify which subcategory of living factor caused the damage, a close examination of the symptoms and signs is required.
Symptoms are the modified appearance of the affected plant, for example necrotic tissues, chlorosis, cankers, galls, leaf distortion.
Signs are the presence of the actual organism or evidence directly related to it: visual observation of the insect on the leaf, presence of fungal mycelium, spores, insect egg masses, insect frass, mite webbing, etc. Signs can be used as clues in identifying the specific living organism that produced the plant damage.
A combination of clues from both symptoms and signs are required for preliminary distinction between pathogen and insect-mite damage.
Symptoms and Signs of Pathogens: Differentiating between bacterial and fungal pathogens is not always clear cut.
Table 6-1: Symptoms and signs of fungal and bacterial leaf spots
| Abnormality | Fungal | Bacterial |
|---|---|---|
| Water-Soaking | Uncommon | Common |
| Texture | Dryish-papery | Slimy-sticky |
| Odor | Usually none | Fishy, rotten |
| Pattern | Circular with concentric rings | Irregular-angular; initially does not cross veins |
| Disintegration | Uncommon | Common |
| Color Changes | Common: red, yellow, purple halos | Uncommon |
| Pathogen Structures | Common -mycelia, spores, etc. | Uncommon |
Fungal diseases
Fungal leaf spots and stem rots are characterized by various symptoms: dry texture, concentric rings, discoloration, and fruiting structures. Fungal leaf spots and stem rots are usually dry or papery. This is especially true in dry climates. The most distinguishing clue of a fungal disease is the presence of signs: mycelium and fruiting bodies of the fungus itself. The fruiting bodies range in size from microscopic to those easily detected with the naked eye. They are found within the leaf spot or stem rot area. Each type of fungus has its own characteristic structures which enable plant pathologists to identify them.
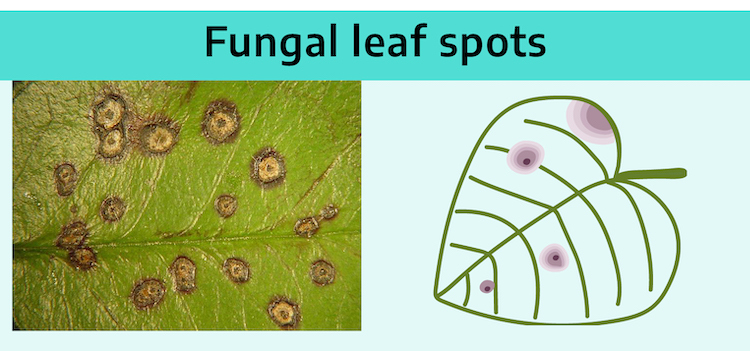
Zones of different color or texture may develop, giving the spot a bull’s eye effect. The dead tissue (tan) is in the center of the spot where the fungal spore germinated. Then as the fungal mycelium front moves outward from the point of dead tissue to healthy, not yet infected tissue on the perimeter, the foliage color changes from dead tan in the center to healthy green on the perimeter.
Spots are not limited by leaf veins since mycelium grows on leaf surface.
Foliar pathogens: The leaf spots caused by fungi generally have distinct margins. Many times they are circular with concentric rings resulting from growth of the mycelium from the center point of initial infection outward (much like crocheting a doily). The condition of the leaf tissue and associated color ranges from dead (necrotic tan) in the center, to recently dead (darker brown ring), to dying (darker ring with possible light yellow, chlorotic edge indicating the advancing edge of the fungal infection). The margins of fungal leaf spots (Figure 6-6) and stem rots can be brightly discolored, such as purple (Fusarium stem rot) or yellow (Helminthosporium leaf spot), making these symptoms quite striking.
Root and stem Pathogens: Root rot and vascular wilt result from fungal infection and destruction of root and stem tissues. The most common visual symptom is gradual wilting of the above-ground shoots.
Bacterial diseases
Bacteria do not actively penetrate healthy plant tissue like fungi. They enter through wounds or natural openings such as leaf stomata or twig lenticels. Once bacteria enter the plant, they reproduce rapidly, killing the plant cells.
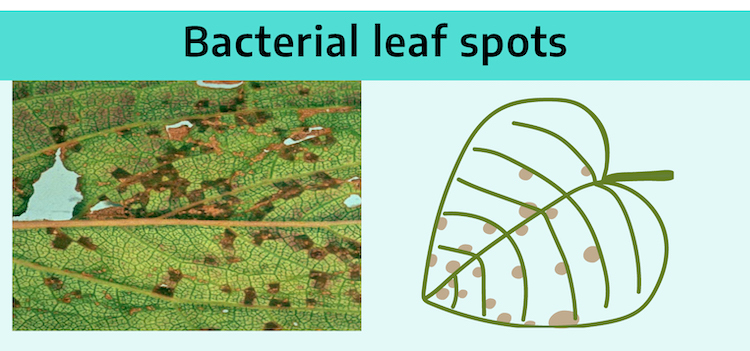
Bacterial leaf spots are often angular because they are initially limited by the leaf veins.
Color of the bacterial spots is usually uniform. Bacteria are one-celled organisms that kill as they go. Tissue may first appear oily or water-soaked when fresh, but upon drying becomes translucent and papery tan.
Bacterial galls: In some cases, toxic materials are produced that cause plant tissues of roots, stems, or leaves to grow abnormally as in crown gall.
Bacterial leaf spot disease: The bacteria usually enter through leaf stomata. Symptoms include water-soaking, slimy texture, fishy or rotten-odor, confined initially between leaf veins resulting in discrete spots that have straight sides and appear angular. Many bacterial leaf spots, such as Xanthomonas leaf spot on Philodendron (also called red edge disease), expand until they reach a large leaf vein. This vein frequently acts as a barrier and inhibits the bacteria from spreading further. A chlorotic halo frequently surrounds a lesion. Lesions may enlarge through coalescence to develop blight lesions. Some lesions exude fluid containing bacteria. Water-soaking frequently occurs in bacterial leaf spot diseases such as Erwinia blight of Dieffenbachia. Holding the leaf to light usually reveals the water-soaking. The ability of bacteria (usually Erwinia species) to dissolve the material holding plant cells together results in a complete destruction of leaf or stem integrity. Some fungi also produce this symptom but not usually as extensively as Erwinia. In general, bacterial infections show this characteristic more than fungal infections. In final stages, cracks form in the tissue and disintegration follows.
Vascular wilt: In some cases the bacteria poison or plug the water conducting tissue and cause yellowing, wilting, browning, and dieback of leaves, stems, and roots.
Viral diseases
Viruses are “submicroscopic” entities that infect individual host plant cells. Once inside a plant cell they are able to infect other cells. Viruses are obligate parasites. They can only replicate themselves within a host’s cell. Because the virus commandeers the host cell to manufacture viruses identical to itself, the plant cell is unable to function and grow normally. In the virus infected plant, production of chlorophyll may cease (chlorosis, necrosis); cells may either grow and divide rapidly or may grow very slowly and be unable to divide (distortion, stunting).

Vein clearing (chlorosis) with interveinal tissue remaining green usually indicates a virus disease or uptake and xylem translocation of a herbicide such as diuron. This is in contrast to the leaf veins remaining green with surrounding chlorotic tissue associated with nutrient deficiencies such as iron deficiency.
Mosaic is a patchwork of green and yellow areas over the surface of a leaf. The leaf may also be puckered and distorted. These symptoms usually indicate a virus disease, especially if yellow areas blend gradually into green areas. If margins are distinct, mottling may indicate a nutritional problem or genetic variegation.
The symptoms of most virus diseases can be put into four categories:
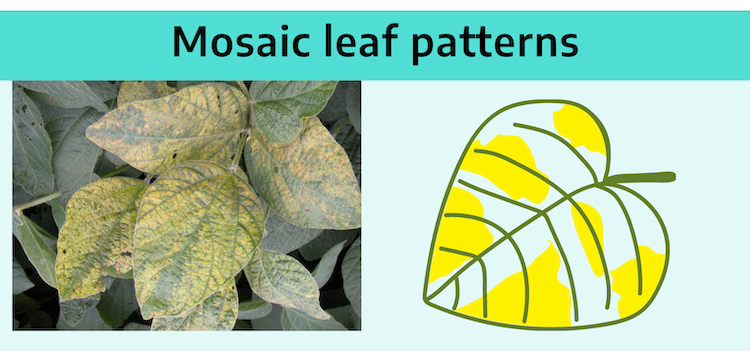
1. Lack of chlorophyll formation in normally green organs:
Foliage may be mottled green and yellow, mosaic, or ringed (yellow or other pigmented ring patterns), or be a rather uniform yellow (virus yellows).
Veins: Vein clearing is a common first symptom of some viral diseases. The veins have a somewhat translucent or transparent appearance. In vein banding there is a darker green, lighter green or yellow band of tissue along the veins.
2. Stunting or other growth inhibition: The reduction in photosynthesis due to less chlorophyll leads to shorter internodes, smaller leaves and blossoms, and reduced yield.
3. Distortions of leaves and flowers: Witches’ brooms or rosettes result from nonuniform growth within a tissue or uncontrolled growth.
4. Necrotic areas or lesions: Being obligate parasites, viruses require the survival of their host plant for their own procreation. Hence, viruses rarely cause death. Necrosis that does occur is usually confined to discrete areas of the plant; necrosis rarely occurs to such an extent that the entire plant is killed.
Viruses typically discolor, deform, or stunt plants rather than induce necrosis or cause death. Expressed symptoms (chlorosis, stunting, distortions) can be valuable clues for virus identification, but can be easily confused with symptoms induced by other problems such as nutritional disorders, spray injuries, or certain feeding damage induced by mites or insects. In addition, because of their extremely small size, the virus or signs of the virus are not visible to the unaided eye. The virus particles are detectable within the plant cell through the electron microscope.
Viruses are transmitted from plant to plant by insects, mites, fungi and nematodes, rubbing, abrasion, or other mechanical means (including grafting or other forms of vegetative propagation). Viruses are occasionally transmitted in seed. Because of the nature of virus transmission, virus symptoms generally spread with time from one infected plant tissue to other plant tissues or from one infected plant to other plants in the community.
Nematodes
Plant nematodes are microscopic roundworms that damage plant tissues as they feed on them. Many feed on or in root tissues. A few feed on foliage or other above-ground organs.
Shoot nematodes: (Aphelenchoides spp.): Foliar nematodes feed inside leaves between major veins causing chlorosis and necrosis. Injury is most often seen at the base of older foliage. When plants with a net-like pattern of veins become infested with foliar nematodes, the tissues collapse in wedge-shaped areas and then change color.
Root nematodes: The most common above-ground symptoms caused by root-infesting nematodes result from damaged root systems. Moisture and nutrient stress symptoms and general stunting are common. The root lesion nematodes (Pratylenchus spp.) and burrowing nematodes (Radopholus similis) destroy the root cortex tissues as they feed. The root-knot nematodes (Meloidogyne spp.) inject growth-regulating substances into root tissues as they feed, stimulating growth of large tender cells to provide themselves a permanent feeding site, and causing overgrowth of root tissues around them to form visible, swollen galls or knots. Other root nematodes stunt growth, apparently by killing root meristems.
Symptoms and Signs of Insects, Mites, and Other Animals
Insects
The location of the feeding damage on the plant caused by the insect’s feeding, and the type of damage (damage from chewing or from sucking mouth parts) are the most important clues in determining that the plant damage is insect-caused and in identifying the responsible insect.
An insect’s life cycle (complete or incomplete) is important when attempting to detect the insect or design a control program.
Feeding habits
Chewing damage or rasping damage
- Entire leaf blade consumed: Entire leaf blade may be consumed by various caterpillars, canker worms, and webworms. Only tougher midvein remains.
- Distinct portions of leaf missing: Distinct notches cut from leaf margin (black vine weevil adult), circular holes cut from margin of leaf (leaf cutter bees), small randomly scattered holes in leaf (beetles, chafers, weevils, grasshoppers).
- Leaf surfaces damaged: “Skeletonization” of leaf surface. Slugs, beetle larvae, pear slug (pear sawfly larvae), elm leaf beetle, and thrips.
- Leaves “rolled”: Leaves that are tied together with silken threads or rolled into a tube often harbor leafrollers or leaftiers (e.g., omnivorous leaftier, Cnephasia longana).
- Leaf miner damage: Leaf miners feed between the upper and lower leaf surfaces. If the leaf is held up to the light, one can see either the insect or frass in the damaged area (discolored or swollen leaf tissue area). Examples: boxwood, holly, birch, elm leaf miners.
- Petiole and leaf stalk borer damage: Petiole and leaf stalk borers burrow into the petiole near the blade or near the base of the leaf. Tissues are weakened and leaf falls in early summer. Sectioning petiole reveals insect-larva of small moth or sawfly larva. Examples: maple petiole borer.
- Damage from twig girdlers and pruners: Twig girdlers (like the twig girdling beetle) may chew all the way around (or girdle) branches of the host plant. Pruners like the vine weevil may eat irregular holes in plants.
- Borer damage: Borers feed under the bark in the cambium tissue or in the solid wood or xylem tissue. Examples: Mountain pine beetle and smaller European elm bark beetle galleries. Damage is often recognized by a general decline of the plant or a specific branch. Close examination will often reveal the presence of holes in the bark, accumulation of frass or sawdust-like material or pitch. Examples: raspberry crown borer, Sequoia pitch moth.
- Root feeder damage: Larval stages of weevils, beetles, and moths cause general decline of plant, chewed areas of roots. Examples: sod webworm, Japanese beetle, root weevil.
Sucking damage

In addition to direct mechanical damage from feeding, some phloem-feeding insects cause damage by injecting toxic substances when feeding. This can cause symptoms which range from simple stippling of the leaves to extensive disruption of the entire plant. Insect species which secrete phytotoxic substances are called toxicogenic (toxin-producing) insects. The resulting plant damage is called “phytotoxemia” or “toxemia.”
- Spotting or stippling: Results from little diffusion of the toxin and localized destruction of the chlorophyll by the injected enzymes at the feeding site. Aphids, leafhoppers, and lygus bugs are commonly associated with this type of injury.
- Leaf curling or puckering: More severe toxemias such as tissue malformations develop when toxic saliva causes the leaf to curl and pucker around the insect. Severe aphid infestations may cause this type of damage.
- Systemic Toxemia: In some cases the toxic effects from toxicogenic insect feeding spread throughout the plant, resulting in reduced growth and chlorosis. Psyllid yellows of potatoes and tomatoes and scale and mealy bug infestations may cause systemic toxemia.
- General (uniform) “stipple,” flecking, or chlorotic pattern on leaf: Examples: adelgid damage on spruce needles and bronzing by lace bugs.
- Random stipple pattern on leaf: Examples: leafhoppers, mites.
- Leaf and stem “distortion”: Associated with off-color foliage = aphids (distortion often confused with growth regulator injury). Examples: rose aphid, black cherry aphid, leaf curl plum aphid.
- Galls, swellings: May occur on leaf and stem tissue and may be caused by an assortment of insects. Examples: aphids, wasps, midge, mossyrose gall wasp, poplar petiole gall midge, azalea leaf gall.
- Damaged twigs are split: Damage resembling split by some sharp instrument is due to egg laying (oviposition) by sucking insects such as tree hoppers and cicadas. Splitting of the branch is often enough to kill the end of the branch. Examples: cicada.
- Root, stem, branch feeders: General decline of entire plant or section of a plant as indicated by poor color, reduced growth, dieback. Examples: Scales, mealybugs, pine needle scale.
Insect life cycles
Knowledge of life cycles assists in identifying the damaging insect.
Incomplete life cycle: Insects resemble the adult upon hatching, except they are smaller and without wings. As the insect grows, it sheds its skin or molts, leaving cast skins as a diagnostic sign. The adult stage is most damaging. Lygus bugs, leafhoppers, and grasshoppers are examples of insects with incomplete life cycles.
Complete life cycle: Eggs, larva (wormlike or grub-like creature that may feed on various plant parts), pupa (relatively inactive, often enclosed in some form of cocoon), and adult insect are completely different in appearance. The larval stage with chewing and rasping feeding is most damaging. Examples of insects with complete life cycles are butterflies, moths, weevils, beetles, and flies.
Other animal damage
Arachnids have sucking mouth parts and 8 legs instead of 6 like the insects. Spider mites have an incomplete life cycle (mite resembles adult throughout life cycle). Damage is often a characteristic stipple pattern on leaf which then becomes pale color on underside (severe infestation causes leaf bronzing and death). Presence of “dirty” foliage indicates small fine webbing on the underside of the foliage mixed with eggs and frass. Eriophyid mites cause distorted new growth, leaf margins roll, leaf veins swell, and distort the leaf (symptoms often confused with growth regulator damage).
Crustacea: Sow bugs and pill bugs feed on decaying vegetation. Not considered to be damaging to live plants.
Mollusca: Slugs and snails. Feeding injury to low-growing foliage resembles skeletonizing or actual destruction of soft tissue. Signs: Presence of ‘silvering’ and slime trails on foliage.
Miscellaneous animals: Millipedes and centipedes (arthropods) feed on decaying plant vegetation (many small legs, brownish or white in color, vary in size from 1/2 – 2”). Not considered to be injurious to live plants.
Small mammals: Chewing of bark and cambium tissue on small trees and shrubs is most frequently by rodents (mice, rabbits, squirrels, and possibly beavers). Signs: Note teeth marks.
Large mammals: Branches torn or clean cut by cattle, goats, deer, and horses.
Birds: Yellow-bellied sap-sucker (even rows of holes in the tree trunk). Missing flower petals, puncture splitting of bark.
Distinguishing Among Nonliving Factors
If patterns of damage in the field planting and on the individual plant are uniform and repeated, this indicates that a nonliving factor is the probable cause of the damage. We will now examine additional information and clues to determine whether the nonliving damaging factor was a mechanical, physical, or chemical factor.
Look for changes in the three categories of nonliving factors of the affected plant’s environment:
- Mechanical Factors – (damage/breakage) – plant damage caused by site changes – “construction damage,” transplanting damage, “lawn mower blight,” abrasion, bruising.
- Physical Factors – environment or weather changes causing extremes of temperature, light, moisture, aeration.
- Chemical Factors – chemical pesticide applications, aerial and soil pollutants, nutritional disorders.
Mechanical factors
Close visual examination and questioning will often determine if the stems or roots have been broken or girdled or if the leaves have been bruised, punctured, or broken. For example, if a large Ficus elastica is dropped while being transplanted and the stem is broken, rapid wilting of the portion of the plant above the break will occur. Examine the plant site for signs of recent excavation, construction, paving, etc.
Physical factors (environmental factors)
Primary sources of diagnostic information are damage patterns and weather records to pinpoint time and location of weather extremes. Records are “signs” of the factor that caused the plant damage.
Temperature extremes
Heat: The highest leaf temperatures will occur in the early afternoon when the sun is located in the southwest quadrant of the sky. Therefore, lethal leaf temperatures produced by solar radiation absorption will occur primarily on unshaded leaves on the outer surface of the plant canopy on the southwest side. Portions of leaves shaded by other leaves or leaves on the shaded northeast side may be undamaged. Most severe damage occurs on the leaves most exposed and furthest from the vascular (roots, stem, leaf vein) source of water, on leaves on the outer perimeter of the plant, leaf tips, and interveinal areas. A recognizable pattern related to leaf tissue that would have the highest potential temperature and be most readily desiccated will occur uniformly over all plants in the area.

Cold: Damage will occur on the least hardy plants and will be most severe on the least hardy tissues of those specific plants. In fall acclimation, cold hardiness is first achieved by the terminal buds, and then with time the lower regions achieve hardiness; the branch crotches are often the last tissues to achieve cold hardiness. Generally the root systems will not survive as low a temperature as will the tops – root systems are damaged at higher temperatures than are the tops. On the other hand, after hardiness has been achieved, if warm temperatures induce deacclimation (for example, in the early spring), the terminals (buds) are first to become less cold hardy.
The portion of plant damaged will indicate if low temperature damage occurred before the plant achieved cold hardiness in the fall, or if it occurred after cold hardiness was lost in the spring. Reverse patterns are produced.
On a given structure (like a leaf or bud), the damage will be death of exposed, nonhardy tissues in a recognizable (repeated) pattern. Frost damage to foliage (e.g., conifer needles) in the spring will uniformly kill all needles of a given age from the tip of the needle back toward the stem a given distance on each needle. Frost cracks are longitudinal separations of the bark and wood generally on the southwest sides of the trunk -most likely to occur because of daily, wide temperature fluctuations. Freezing death of dividing cells on outer portions of leaf folds inside the bud will cause distorted or lace-like leaf blade because of non-uniform cell division and growth during leaf expansion. Cold damage to the root system is primarily a concern with container-grown plants where the root temperature fluctuates more and can be expected to reach lower temperatures than would occur with the same plant if field-grown. Cold damage to the root system can be detected by examining the roots. Damage generally occurs from the periphery of the root ball (near the container edge) and evidence includes blackened or spongy roots with lack of new growth or new root hairs. Above ground symptoms generally will not be evident until new shoot growth in the spring; at that time leaf expansion may be incomplete (small leaf size) because of the restricted uptake of water and nutrients by the damaged root system. With increased air temperatures, the water loss from the shoots and leaves may exceed the root uptake capacity, and the plants may defoliate due to this water deficiency.
Plants Vary in their Cold Tolerance: The cold tolerance (hardiness) of various plants in the landscape has been rated by the USDA. The most recent (2012) version is based on weather data from 1976–2005. To check your USDA hardiness zone, check the USDA Plant Hardiness Zone Map.
Light extremes
Plants can acclimate to various conditions, but the primary requirement for acclimation is time. Plants respond adversely to rapid changes in the environment. Rapid change from low to high light intensity will result in destruction of the chlorophyll pigments in the leaf (yellowing and necrosis = sunburn). Rapid change from high to low light intensity will result in reduced growth and leaf drop; new leaves will be larger. “Sun leaves” are smaller, thicker and lighter green in color than are “shade leaves.” Flowering will be reduced, delayed, or absent under low light.
Oxygen and moisture extremes
Here we are primarily considering the root environment where oxygen and moisture are inversely related. Water logging (moisture saturation) of the root environment results in oxygen deficiency. Without oxygen, root metabolism and growth come to a standstill. Consequently, uptake of water and nutrients is restricted with subsequent wilting and nutritional deficiency symptoms occurring on the above-ground portions of the plant. Drought and water logging produce many of the same symptoms on the above-ground portion of the plant. The first symptoms will be chlorosis and abscission of older leaves. Under severe, continuing moisture stress, wilting and necrosis will occur on tips and interveinal regions of recently expanded leaves and new growth.
Chemical factors
Chemical injury patterns on an individual plant
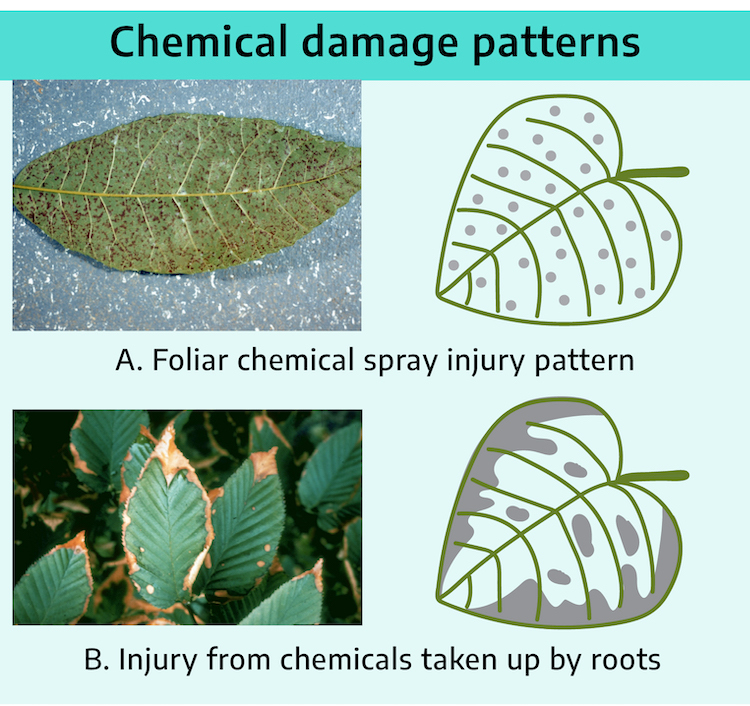
A general uniform pattern of damage occurring over several plant species and over a relatively large area indicates a nonliving factor such as a chemical phytotoxicity. Questions-answers, records, the plant symptoms, and knowledge about the mobility within the plant of the common chemicals (nutrients and pesticides) should help determine which chemical caused the damage.
Patterns of injury symptoms on an individual plant that develop because of deficiency, excess, or toxicity of a chemical differ, depending primarily upon whether the chemical causes damage directly on contact or is absorbed and distributed within the plant through phloem-translocation or through xylem-translocation.
Symptoms from direct contact of chemicals with the plant
Shoot foliage contact: Symptoms from shoot contact chemicals occur over the general plant canopy. If the toxic chemical is applied directly to the above-ground parts of the plant (shoot-foliage contact chemical), the physical pattern of application may be detected (e.g., spray droplet size, etc.). If the toxic chemical is spray-applied, the pattern of spray droplets or areas where spray accumulated to runoff along the leaf edges will show the most severe damage. If it is a toxic gas (a volatile chemical acting as an aerial pollutant), the areas between the leaf veins and along the leaf margins where the concentration of water within the leaf is lower will be the first to show damage. Injury from foliar applications of insecticides, fungicides, and fertilizers is primarily of the direct-contact type and is typified by chlorotic-necrotic spotting, especially interveinally and along leaf edges and other areas where chemical concentrates and is least diluted by inter-cellular moisture. Examples of shoot-foliage contact chemicals are foliar-applied fertilizer salts and the herbicides paraquat, acifluorfen, dinoseb, and herbicidal oils.
Root contact: Toxic contact chemicals in the root zone, including excess fertilizer, result in poor root development. Symptoms from root-contact chemicals are localized where the chemical contacts the root, but produce general symptoms in the shoot. The shoots may show water and nutrient stress symptoms (e.g., reduced growth, wilting, nutrient deficiency symptoms). The injury symptoms on the shoot and foliage from root damage by direct contact with toxic chemicals or excessive salts resembles a drying injury; the roots are unable to obtain water. Roots are injured and root tips may be killed. This will result in a general stunting of the plant. In severe cases, wilting can occur even though the soil is wet. Lower leaves generally wilt first and this is followed by a marginal drying of the leaves. Many factors injuring or inhibiting root growth may produce similar shoot symptoms. Nematodes, soil compaction, cold weather, salinity, nutritional disorders, and certain herbicides (dinitroanilines, DCPA, and diphenamid) cause root inhibition.
Symptoms of deficient or toxic translocated chemicals
The effects of mobile chemicals absorbed by the plant are dependent upon whether the chemical is transported in the phloem or in the xylem. If transported solely in the xylem system, the chemical will move upward in the plant in the xylem-transpiration stream. Toxic symptoms from xylem-translocated chemicals occur primarily in the older foliage. Deficiency symptoms of xylem-transported (phloem-immobile) nutrient ions will occur first in the new growth.
If the chemical is translocated in the phloem, it may move multidirectional from the point of absorption (i.e., it may move from the shoot to the root or the reverse). Toxic symptoms from phloem-translocated chemicals occur primarily in the new growth and meristematic regions of the plant. Deficiency symptoms of phloem-retranslocated nutrient ions occur first in the older foliage.
Xylem translocated chemicals move primarily upward in the plant to the foliage. A chemical is translocated upward in the xylem (apoplastic movement) of the plant from the point of absorption. Symptoms occur in tissues formed after the toxicity or deficiency occurs.
Toxic chemicals, xylem translocated: When toxic chemicals are translocated to fully expanded, older leaves, the toxicity symptoms generally appear on the leaf margins and interveinal areas. When toxic chemicals are translocated to immature, young leaves, the toxicity symptoms generally appear associated with the veins, especially the midrib.
- Photosynthetic-inhibiting chemicals: Injury from translocated toxic chemicals is primarily to the foliage. Plant injury generally progresses from the lower, older foliage to the top. Individual leaves show greatest injury (chlorosis) along their tips and margins or along the veins. Examples of xylem-translocated herbicides include the photosynthetic inhibitors such as the triazine, urea, and uracil herbicides.
- Shoot-inhibiting chemicals: Examples of toxic chemicals absorbed by the roots and translocated in the xylem to the shoots are the “shoot inhibiting herbicides.” The shoot inhibitors cause malformed and twisted tops with major injury at the tips and edges of the leaves; looping of the leaves may occur since the base of the leaf may continue to grow while the leaf tips remain twisted together. Thiocarbamate herbicides cause these symptoms on both grasses and broadleaves. Alachlor and metolachlor herbicides cause similar injury symptoms on grasses.
Deficient nutrient Ions, xylem-translocated (phloem immobile): Several nutrient ions are immobile after upward translocation in the xylem and incorporation in plant tissue. They cannot be withdrawn when deficiencies develop in the root zone and retranslocated in the phloem to the new growth. Deficiency symptoms of phloem-immobile nutrient ions develop on the new growth. Boron and calcium are quite phloem-immobile, which means that if the external supply becomes deficient, the symptoms of boron and calcium deficiency will appear first on the new growth. And, with severe deficiencies, the terminal bud dies. Iron, manganese, zinc, copper, and molybdenum are also relatively phloem-immobile and are not readily withdrawn from the older leaves for translocation through the phloem to younger leaves and organs. Deficiency symptoms are most pronounced on the new growth. Phloem-translocated chemicals move multidirectionally from point of application or source of the chemical to the meristematic regions.
Toxic chemicals, phloem translocated: Injury from phloem-translocated toxic chemicals is primarily to new leaves and roots because of translocation of chemical to the meristems. Whether taken up by the roots or shoots, these compounds are moved through the living plant cells and phloem (symplastic movement) to both the root and shoot tips. The young tissue (shoots or roots) will be discolored or deformed and injury may persist for several sets of new leaves. Examples of phloem-translocated toxic chemicals, whether absorbed by the roots or shoots, include the herbicides 2,4-D, dicamba, picloram, glyphosate, amitrole, dalapon, sethoxydim, and fluazifopbutyl. These compounds move to the meristems and typically injure the youngest tissues of the plant.
Deficient nutrient ions, phloem mobile: If phloem mobile nutrient ions become deficient in the root zone, these ions may be withdrawn from the older plant tissue and retranslocated in the phloem to the new growth. In such situations, deficiency symptoms will first occur on the older leaves. Elements that may be withdrawn from older leaves and retranslocated in the phloem to younger leaves and storage organs include nitrogen, phosphorus, potassium, magnesium, chlorine, and in some plant species, sulfur. In plant species where sulfur can be withdrawn from the older leaves and translocated to the newer growth, deficiency symptoms may initially occur on the older leaves or over the plant in general. In plants where sulfur is not readily retranslocated, the older leaves may remain green and the sulfur deficiency symptoms occur only on the new growth.
Key to symptoms of chemical disorders
I. Symptoms appearing first or most severely on new growth (root and shoot tips, new leaves, flowers, fruits, buds).
A. Terminal bud usually dies. Symptoms on new growth:
- Boron deficiency: Basal part of young leaves and internal tissues of organs may become necrotic. One of the earliest symptoms is failure of the root tips to elongate normally. Terminal shoot meristems also die giving rise to a witch’s broom. Young leaves become very thick, leathery, and chlorotic; in some species young leaves may be crinkled because of necrotic spots on leaf edge during development. Young leaves of terminal buds become light green then necrotic and stem finally dies back at terminal bud. Rust colored cracks and corking occur on young stems, petioles, and flower stalks. “Heart rot” of beets, “stem crack” of celery.
- Calcium deficiency: Necrosis occurs at tip and margin of leaves, causing a definite hook at leaf tip. Calcium is essential for the growth of shoot and root tips (meristems). The growing point dies. Margins of young leaves are scalloped and abnormally green and, due to inhibition of cell wall formation, the leaf tips may be “gelatinous” and stuck together, inhibiting leaf unfolding. Stem structure is weak and peduncle collapse or shoot topple may occur. Roots are stunted. Premature shedding of fruit and buds is common. Downward curl of leaf tips (hooking) occurs near terminal bud. Ammonium or magnesium excess may induce a calcium deficiency in plants.
- Differentiating between calcium and boron deficiency symptoms: when calcium is deficient, there is a characteristic hooking of the youngest leaf tips. However, when boron is deficient, the breakdown occurs at the bases of the youngest leaves. Death of the terminal growing points is the final result in both cases.
- Ammonium excess: Tissue breakdown – necrosis and firing of the tip and margins of the leaf. The ammonium cation in itself may become phytotoxic and result in breakdown of the plant tissue (proteolysis breakdown of plant proteins) initially producing a wet, dark-green, “steamed” appearance at the leaf tips and margins. This destroyed tissue eventually desiccates and becomes a light tan color. Excess ammonium may also induce calcium deficiency (abnormally dark green foliage, scalloped leaf margins, weak stem structure, death of terminal bud or growing point of the plant, premature shedding of the blossoms and buds).
B. Terminal bud remaining alive. Symptoms on new growth.
Interveinal chlorosis on young leaves:
- Iron deficiency: Interveinal chlorosis on young leaves with larger veins only remaining green. Necrotic spots usually absent; however, with extreme deficiencies, young leaves are almost white and may have necrotic margins and tips; necrotic spots may extend inward. Potassium, zinc or copper excess can inhibit uptake of iron. High pH may also induce iron deficiency.
- Iron deficiency symptoms are similar to those of magnesium deficiency but iron deficiencies occur in young leaves first. Iron accumulated in older leaves is relatively immobile in the phloem.
-
Manganese deficiency: Interveinal chlorosis with smallest veins remaining green producing a checkered or finely netted effect. Grey or tan necrotic spots usually develop in chlorotic areas; the dead spots of tissue may drop out of the leaf, giving a ragged appearance. Poor bloom–both size and color. Potassium excess can inhibit uptake of manganese.
- Zinc deficiency: Stunted new growth with interveinal chlorosis: young leaves are very small (“little leaf”), sometimes missing leaf blades altogether, and internodes are short, giving a rosette appearance.
Interveinal chlorosis is not the main symptom on new growth:
- Copper deficiency: Wilting and loss of turgor of young, terminal leaves and stem tips is common. Symptoms are highly dependent upon plant species. In some species younger leaves may show interveinal chlorosis while tips and lobes of older leaves remain green followed by veinal chlorosis and rapid, extensive necrosis of leaf blade.
- There are no known reports of H2PO4 toxicity; however, plants may take up the phosphate anion in luxury amounts.
- Phosphorus excess: Phosphorus excess is associated with impeded uptake and possible deficiency of copper and sometimes of zinc.
- Sulfur deficiency: Leaves light green, veins lighter in color than adjoining interveinal areas. Leaves over entire plant may become yellowish-green, roots and stems are small in diameter and are hard and woody. Young leaves may appear to be uniformly yellow. Some necrotic spots.
- Xylem- translocated “shoot-inhibiting chemicals”: Shoot inhibition causing malformed and twisted tops with major injury at the tips and edges of the leaves.
- Examples of toxic xylem-translocated chemicals include the thiocarbamate herbicides (symptoms on grasses and broadleaves) and alachlor and metolachlor (symptoms on grasses).
- Xylem- translocated chemicals: Young tissues discolored or deformed and injury may persist for several sets of new leaves.
- Examples of toxic phloem-translocated chemicals include the herbicides 2, 4-d, dicamba, picloram, glyphosate, amitrole, dalapon, sethoxydim, and fluazifopbutyl.
II. Symptoms do not appear first or most severely on youngest leaves: effect general on whole plant or localized on older, lower leaves.
A. Chlorosis general, no interveinal chlorosis. Effects usually general on whole plant.
- Nitrogen deficiency: Visible symptoms include yellowing and dying of older leaves. Foliage light green, growth stunted, stems slender, yellow.
- Plants receiving enough nitrogen to attain limited growth exhibit deficiency symptoms consisting of a general chlorosis, especially in older leaves. In severe cases, these leaves become completely yellow and then light tan as they die. They frequently fall off the plant in the yellow or tan stage.
- Zinc excess: Older leaves wilt. Entire leaf is affected by chlorosis, but edges and leaf tissues near main veins often retain more color (chlorophyll).
B. Vein-clearing, chlorosis-necrosis at leaf tips and margins on older-younger foliage.
- Xylem- transported photosynthetic- inhibitors: When toxic chemicals are xylem-translocated to older, fully-expanded leaves, the toxicity symptoms generally occur on the margins and interveinal areas of the leaf. When translocated to young, expanding leaves, toxicity symptoms are generally associated with the veins, especially the midrib. Examples of xylem-translocated, photosynthetic inhibitors include the triazine, urea, and uracil herbicides.
C. Interveinal chlorosis. Interveinal chlorosis first appears on oldest leaves.
- Magnesium deficiency: Older leaves chlorotic, usually necrotic in late stages. Chlorosis along leaf margins extending between veins produces a “Christmas tree” pattern. Veins normal green. Leaf margins may curl downward or upward with puckering effect. Necrosis may suddenly occur between veins. Potassium or calcium excess can inhibit uptake of magnesium.
- When the external magnesium supply is deficient, interveinal chlorosis of the older leaves is the first symptom because as the magnesium of the chlorophyll is remobilized, the mesophyll cells next to the vascular bundles retain chlorophyll for longer periods than do the parenchyma cells between them. Leaves lose green color at tips and between veins followed by chlorosis or development of brilliant colors, starting with lower leaves and proceeding upwards. The chlorosis/brilliant colors (unmasking of other leaf pigments due to the lack of chlorophyll) may start at the leaf margins or tips and progress inward interveinally producing a “Christmas tree” pattern. Leaves are abnormally thin, plants are brittle, and branches have a tendency to curve upward. Twigs are weak, subject to fungus infection, usually leaves drop prematurely; plant may die the following spring.
- Manganese excess: Smaller veins in older leaves may turn brown. Small necrotic spots in older leaves spread margins inwards, and finally desiccate the entire leaf blade. At severe, advanced stages, young leaves also display this spotting.
- Molybdenum deficiency: Chlorotic areas (pale yellow) on whole plant; leaf edges curl upwards. General symptoms are similar to those of nitrogen deficiency. Interveinal chlorosis occurring first on the older or midstem leaves, then progressing to the youngest. Sometimes, as in the “whiptail” disease, plants grown on ammonium nitrogen may not become chlorotic, but develop severely twisted young leaves which eventually die. Other characteristic molybdenum deficiency symptoms include marginal scorching and rolling or cupping of leaves. With molybdenum deficiency, nitrogen deficiency symptoms may develop in the presence of adequate levels of nitrate nitrogen in the root environment and high levels of nitrate nitrogen in the plant. Nitrate nitrogen must be reduced in the plant before it can be utilized. Molybdenum is required for this reduction, and if it is deficient, nitrate may accumulate to a high level in the plant, yet at the same time the plant may exhibit nitrogen deficiency symptoms. Molybdenum differs from other trace nutrients in that many plants can develop in its absence, provided that ammonium nitrogen is present. Molybdenum appears to be essential for the nitrate-reducing enzyme to function.
- Fluoride, Fluorine excess: Foliar marginal necrosis is the most common symptom of fluoride toxicity along with chlorosis. Chlorosis along and between the veins occurs in fluorine-sensitive plants. With many plants, the marginal necrosis is preceded by the appearance of gray or light-green, water-soaked lesions which later turn tan or reddish-brown. Injury generally occurs at the tips of the leaves first, then moves inward and downward until a large part of the leaf is affected.
D. Leaf chlorosis is not the dominant symptom. Symptoms appear on older leaves at the base of the plant.
Plant dark green:
- Phosphorus deficiency: At first, all leaves are dark green and growth is stunted. Purple pigment often develops in older leaves, particularly on the underside of the leaf along the veins. Leaves drop early. Phosphorus deficiency is not readily identified by visual symptoms alone. Visual symptoms of phosphorus deficiency are not always definite, but many phosphorus deficient plants exhibit off-color green foliage with purple venation, especially on the underside of leaves, and plants are stunted and remain stunted even when fertilizers supplying potassium and nitrogen are applied. Older leaves assume a purple-bronze color. Small growth, especially root development; spindly growth with tips of older leaves often dead. Phosphorus is retranslocated by the phloem from older leaves to new growth.
- Aluminum excess: Aluminum appears to affect root growth in particular: root tips blacken, no longer lengthen, but become thickened. Excess aluminum accumulation in roots reduces their capacity for translocating phosphorus. Amelioration involves suppression of aluminum activity, for example by liming to bring the medium’s pH above 5.5, and not by addition of phosphorus. The toxic amount of aluminum in a soil will depend upon other soil properties such as pH and phosphorus content and upon the plant grown. Media amendments such as perlite may release toxic quantities of aluminum if the media pH is extremely acid.
Leaves are thick , brittle, and deep green: (In acute toxicity, older leaves wilt and scorch from the margins inward)
- Nitrate excess
Necrotic spots develop on older leaves:
- Potassium deficiency: Margins of older leaves become chlorotic and then burn, or small, chlorotic spots progressing to necrosis appear scattered on old leaf blades. Calcium excess impedes uptake of potassium cations.
- Potassium deficiency symptoms first appear on the recently matured leaves of the plant (not on the young, immature leaves at the growing point). In some plants, the first sign of potassium deficiency is a white specking or freckling of the leaf blades. With time, the symptoms become more pronounced on the older leaves, and they become mottled or yellowish between the veins and scorched at the margins. These progress inward until the entire leaf blade is scorched. If sodium cations are present and taken up in place of K+, leaf flecking (necrotic spots scattered on leaf surface) and reduced growth occur. Seed or fruit is shriveled. Potassium is retranslocated by the phloem from old leaves to new growth.
- Boron excess: Tips and edges of leaves exhibit necrotic spots coalescing into a marginal scorch. Symptom from the plant’s base upwards with older leaves being affected first. In advanced, severe toxicity, necrotic spots with a pale brown center also appear in the inner parts of the leaf blade.
- Direct-contact of toxic chemical with shoot and foliage: Mottling and necrotic spots primarily on margin and interveinally may be due to excessive amounts of fertilizers or pesticides applied as foliar sprays. Examples of shoot direct-contact toxic chemicals include the shoot-foliage applied herbicides paraquat, acifluofen, dinoseb, and the herbicidal oils which produce this type of symptom.
- Direct-contact injury by toxic chemicals (or other factors in the root zone, e.g., low temperatures, nematodes, root weevils): Reduced growth and wilting of older leaves with development of chlorotic and necrotic spots. Roots become stunted in length and thickened, or club-shaped, near the tips: the shoots remain normal but may show nutrient and moisture stress. Under severe conditions, root tips may be killed, causing general stunting of the plant and wilting followed by marginal drying of the lower leaves first. Examples of root direct-contact toxic chemicals include excess salts or presence of toxic chemical such as the herbicides dcpa, dinitroanilines, diphenamid.
- Chloride deficiency: Leaves often eventually become bronze colored.
- Excess salt or sodium excess: Marginal scorching that may progress to general leaf scorching. Generally no spotting.
- Molybdenum excess: Intense yellow or purple color in leaves. Molybdenum excess or toxicity in field grown plants is rarely observed. Plants appear to tolerate relatively high tissue concentrations of molybdenum. Isolated reports of symptoms from excess molybdenum include development of intense yellow color in tomato leaves and intense purple color in cauliflower leaves.
Summary: Systematic approach to diagnosing plant damage
I. Define the problem (determine a “real” problem exists).
- Identify plant and know characteristics. Establish what the “normal” plant would look like at this time of year. Describe the “abnormality”: symptoms and signs.
- Examine the entire plant and its community. Determine the primary problem and part of the plant where initial damage occurred.
II. Look for patterns: On more than one plant? On more than one plant species?
- Understand nonuniform damage pattern (scattered damage on one or only a few plant species) is indicative of living factors (pathogens, insects, etc.).
- Understand uniform damage pattern over a large area (i.e., damage pattern on several plant species) and uniform pattern on the individual plant and plant parts indicates nonliving factors (mechanical, physical, or chemical factors).
- Compare patterns of living and nonliving factors on plant community, plant, plant part.
III. Delineate time-development of damage pattern.
- Progressive spread of the damage on a plant onto other plants or over an area with time indicates damage caused by living organisms.
- Damage occurs, does not spread to other plants or parts of the affected plant. Clear line of demarcation between damaged and undamaged tissues. These clues indicate nonliving damaging factors.
IV. Determine causes of the plant damage. Ask questions and gather information.
- Distinguish among living factors.
- Symptoms and signs of pathogens.
- Symptoms and signs of insects, mites, and other animals.
- Distinguish among nonliving factors.
- Mechanical factors.
- Physical factors.
- Temperature extremes.
- Light extremes.
- Oxygen and moisture extremes.
- Chemical factors.
- Analyze damage patterns in fields and other plantings.
- Injury patterns on individual plants.
- Pesticide-pollutant phytotoxicities – damage, patterns.
- Nutritional disorders-key to nutritional disorders.
- References (check reports of damaging factors on identified plant); may need laboratory analyses to narrow range of probable causes.
V. Synthesis of information to determine probable causes.
Diagnostic Keys
These are useful keys to keep at help desks. Use them to match common problems with their causes and control methods. For specific control recommendations, always refer to the “Pest Management Guide: Home Grounds and Animals” 456-018.
Table 6-2: Vegetable Diagnostic Key
| Crop | Symptoms | Possible Causes | Controls & Comments |
|---|---|---|---|
| All Vegetables | Poor fruit yield; fruit may be small and have poor taste | Uneven moisture | Supply water during dry periods |
| All Vegetables | Poor fruit yield; fruit may be small and have poor taste | Poor soil fertility or improper soil pH | Soil test |
| All Vegetables | Plants grow slowly; leaves light green | Insufficient light | Thin plants; do not plant in shade |
| All Vegetables | Plants grow slowly; leaves light green | Cool weather | |
| All Vegetables | Plants grow slowly; leaves light green | Poor soil fertility or improper soil pH | Soil test |
| All Vegetables | Plants grow slowly; leaves light green | Excess water | Do not overwater; improve drainage |
| All Vegetables | Plants grow slowly; leaves light green | Seed corn maggots | Replant with insecticidal seed treatment |
| All Vegetables | Plants grow slowly; leaves light green | Insufficient water | Supply water |
| All Vegetables | Seedlings don’t emerge | Dry soil | Supply water |
| All Vegetables | Seedlings don’t emerge | Seeds washed away | |
| All Vegetables | Seedlings don’t emerge | Damping-off (fungal or oomycete disease) | Do not overwater; treat seed with registered fungicide |
| All Vegetables | Seedlings don’t emerge | Incorrect planting depth | |
| All Vegetables | Seedlings don’t emerge | Slow germination due to cool weather | Plant when ground is warm |
| All Vegetables | Seedlings don’t emerge | Root maggots | Use registered soil insecticide |
| All Vegetables | Wilted seedlings; seedlings fall over | Dry soil | Supply water |
| All Vegetables | Wilted seedlings; seedlings fall over | Damping-off (fungal or oomycete disease) | Do not overwater; treat seed with registered fungicide |
| All Vegetables | Wilted seedlings; seedlings fall over | Cutworms | Use registered soil insecticide; use plant collars |
| All Vegetables | Wilted seedlings; seedlings fall over | Root maggots | Use registered soil insecticide |
| All Vegetables | Chewed seedlings | Rodents, rabbits, or birds | Fence or netting |
| All Vegetables | Chewed seedlings | Slugs | Use slug bait (beer or commercial slug bait) |
| All Vegetables | Chewed seedlings | Various insects | Submit insect for identification; use registered insecticide for specific insect (see PMG) |
| All Vegetables | Wilted plants; bottom leaves may turn yellow | Dry soil | Supply water |
| All Vegetables | Wilted plants; bottom leaves may turn yellow | Root rot (fungal or oomycete disease) | Do not overwater; remove old plant debris; rotate |
| All Vegetables | Wilted plants; bottom leaves may turn yellow | Vascular wilt (fungal or bacterial disease: mainly affecting tomato, potato, eggplant, pepper) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG; use resistant varieties; rotate |
| All Vegetables | Wilted plants; bottom leaves may turn yellow | Root knot (nematode problem) | Check roots for galls; rotate; grow cover crops antagonistic to nematodes before replanting crop; incorporate organic matter into soil to stimulate microbial activity antagonistic to nematodes; plant French marigolds to infested area for one year and incorporate into soil to reduce nematode populations; use resistant varieties if available |
| All Vegetables | Wilted plants; bottom leaves may turn yellow | Various root-feeding nematodes | Submit soil sample for nematode analysis; rotate; grow cover crops antagonistic to nematodes before replanting crop; incorporate organic matter into soil to stimulate microbial activity antagonistic to nematodes; plant French marigolds to infested area for one year and incorporate into soil to reduce nematode populations; use resistant varieties if available |
| All Vegetables | Wilted plants; bottom leaves may turn yellow | Walnut wilt (mainly affecting tomato) | Rule out vascular wilt disease with laboratory diagnosis; do not plant tomatoes near walnut or butternut trees; sever roots bordering garden and place barrier between tree and garden |
| All Vegetables | Wilted plants; bottom leaves may turn yellow | Waterlogged soil | Improve drainage |
| All Vegetables | Wilted plants; bottom leaves may turn yellow | Insects with sucking mouthparts, such as aphids and true bugs | Submit insect for identification; use registered insecticide for specific insect (see PMG) |
| All Vegetables | General leaf yellowing; no wilting | Nutrient or mineral deficiency; improper soil pH | Soil test |
| All Vegetables | General leaf yellowing; no wilting | Insufficient light | Thin plants |
| All Vegetables | Leaves stippled with tiny white spots | Spider mites | Treat with registered miticide |
| All Vegetables | Leaves stippled with tiny white spots | Air pollution (ozone) | |
| All Vegetables | Leaves stippled with tiny white spots | Leafhoppers, thrips, or aphids | Submit insect for identification; use registered insecticide for specific insect (see PMG) |
| All Vegetables | Leaf margins turn brown and shrivel | Dry soil | Supply water |
| All Vegetables | Leaf margins turn brown and shrivel | Salt damage | Do not place garden where de-icing salt may have been applied on nearby concrete |
| All Vegetables | Leaf margins turn brown and shrivel | Fertilizer burn | Soil test for soluble salts level; do not over-apply fertilizer; flush soil with water |
| All Vegetables | Leaf margins turn brown and shrivel | Potassium deficiency | Soil test |
| All Vegetables | Leaf margins turn brown and shrivel | Cold injury | |
| All Vegetables | Leaf margins turn brown and shrivel | Thrips | Treat with registered insecticide |
| All Vegetables | Leaf margins turn brown and shrivel | Mites | Treat with registered miticide |
| All Vegetables | Discrete brown spots on leaves; some spots may coalesce | Fungal or bacterial leaf spot disease | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| All Vegetables | Discrete brown spots on leaves; some spots may coalesce | Chemical injury from contact burn-type chemical | Do not apply chemicals that are not registered for use on the plant; apply chemicals at registered rates; some chemical injury occurs from drift |
| All Vegetables | Discrete brown spots on leaves; some spots may coalesce | Four-lined plant bugs | Use registered insecticide |
| All Vegetables | White powdery growth on upper and lower leaf surfaces | Powdery mildew (fungal disease) | Use registered fungicide; use resistant varieties |
| All Vegetables | Leaves shredded or stripped from plant | Hail damage | |
| All Vegetables | Leaves shredded or stripped from plant | Rodents | Place fence around garden |
| All Vegetables | Leaves shredded or stripped from plant | Slugs | Use slug bait |
| All Vegetables | Leaves shredded or stripped from plant | Dead tissue dropping out, following fungal or bacterial infection | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| All Vegetables | Leaves shredded or stripped from plant | Various insects | Submit insect for identification; use registered insecticide for specific insect (see PMG) |
| All Vegetables | Leaves with yellow and green mosaic or mottled pattern; leaves may be puckered and plant stunted | Viral disease | Use resistant varieties if available; weed control; remove affected plants; remove old plant debris; insect control |
| All Vegetables | Leaves curled, puckered, or distorted | Herbicide injury from growth regulator-type herbicide (common on tomato, cucumber, bean) | If lawn herbicides are used, apply after wind has died down and do not apply in heat of day; avoid using mulch from herbicide-treated fields, manure from animals fed on herbicide-treated fields or grass clippings from herbicide-treated lawns |
| All Vegetables | Leaves curled, puckered, or distorted | Viral disease | Use resistant varieties if available; weed control; remove affected plants; remove old plant debris; insect control |
| All Vegetables | Leaves curled, puckered, or distorted | Aphids, leafhoppers, or thrips (insects) | Use registered insecticide; keep plants well watered; use reflective mulch |
| Asparagus | Tops turn yellow, brown, and die back; reddish-brown, orange or black pustules appear on stems and leaves | Rust (fungal disease) | Cut tops close to ground in fall and destroy cuttings; use registered fungicide; use resistant varieties |
| Asparagus | Shoots wilt, turn yellow, then brown; roots are reddish color | Fusarium wilt (fungal disease) | Destroy infected plants; rotate for 2-4 years; use resistant varieties |
| Asparagus | Shoots wilt, turn yellow, then brown; roots are reddish color | Root rot (fungal or oomycete disease) | Rotate; remove old plant debris; plant in well-drained area |
| Asparagus | Small spears | Immature plants | Asparagus produces small spears for the first 2-3 years after planting |
| Asparagus | Small spears | Overharvested plants | Do not harvest late into the season; plants cannot store enough carbohydrates for following season |
| Asparagus | Small spears | Poor fertility or improper soil pH | Soil test |
| Asparagus | Small spears | Poor drainage | Do not overwater; plant in well-drained area |
| Asparagus | Spears crooked or bent at tip | Mechanical injury from windblown sand or mishandling | |
| Asparagus | Spears crooked or bent at tip | Insect injury | Control asparagus beetles with registered insecticide |
| Asparagus | Spears crooked or bent at tip | Cold injury | |
| Asparagus | Spears turn brown and soft | Frost injury | Protect spears with mulch on nights when cold temperatures are expected |
| Asparagus | Spears turn brown and soft | Root rot (fungal or oomycete disease) | Remove old plant debris; rotate; plant in well-drained area |
| Asparagus | Leaves chewed; slime may be present on leaves; no evidence of insects | Slugs (emerge at night and hide during the day) | Use slug bait |
| Asparagus | Spears and leaves chewed or scarred | Asparagus beetles | Use registered insecticide |
| Basil | Leaves with irregular browning or yellowing as if declining early; fuzzy, gray growth (looks like soil) may be present on lower leaf surface | Downy mildew (oomycete disease) | Use resistant varieties |
| Basil | Wilt; leaf chlorosis; black streaking on stems | Fusarium wilt (fungal disease) | Remove affected plants; rotate with plants not in the mint family for at least 3 years; avoid high-ammonium fertilizers; varieties with resistance to Fusarium wilt are available, but they are not resistant to downy mildew |
| Bean | Plants wilted or are stunted; leaves may turn yellow | Dry soil | Supply water |
| Bean | Plants wilted or are stunted; leaves may turn yellow | Root rot (fungal or oomycete disease) | Remove old plant debris; rotate; plant in well-drained area |
| Bean | Plants wilted or are stunted; leaves may turn yellow | Root knot (nematode problem) | Check roots for galls; rotate; grow cover crops antagonistic to nematodes before replanting crop; incorporate organic matter into soil to stimulate microbial activity antagonistic to nematodes; plant French marigolds to infested area for one year and incorporate into soil to reduce nematode populations; use resistant varieties if available |
| Bean | Plants wilted or are stunted; leaves may turn yellow | Poor fertility or improper pH | Soil test |
| Bean | Plants wilted or are stunted; leaves may turn yellow | Root maggots | Use registered insecticide |
| Bean | Failure to set pods | High temperatures, causing blossoms to drop | |
| Bean | Failure to set pods | Dry soil | Supply water |
| Bean | Failure to set pods | Wet soil, causing lack of oxygen to roots | Do not overwater; plant in well-drained soil |
| Bean | Failure to set pods | Mature pods left on vines, causing seed production rather than pod set | Pick pods regularly |
| Bean | Rust-colored, powdery spots surrounded by yellow haloes form on leaves, stems, and pods | Rust (fungal disease) | Use resistant varieties; use registered fungicide; remove old plant debris |
| Bean | Soft, watery spots on leaves, stems, and pods; white moldy growth on affected plant parts; plants wilt and die | White mold (fungal disease) | Use registered fungicide; rotate; remove old plant debris |
| Bean | Thin, white powdery growth on leaves and pods | Powdery mildew (fungal disease) | Use resistant varieties; use registered fungicide; rotate; remove old plant debris |
| Bean | Small, brown spots surrounded by yellow haloes on leaves; leaves wither | Bacterial blight | Avoid overhead watering, which spreads the disease; use fixed copper bactericide if available; rotate |
| Bean | Brown spots without yellow haloes appear on leaves, pods and seeds; leaves wither | Fungal disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Bean | Brown spots without yellow haloes appear on leaves, pods and seeds; leaves wither | Stink bugs | Use registered insecticide |
| Bean | Leaves skeletonized; copper colored beetles with black spots or yellow grubs present | Mexican bean beetle | Hand pick beetles and grubs or use registered insecticide |
| Bean | Leaves with white flecks | Spider mites | Use registered miticide |
| Bean | Leaves with white flecks | Thrips | Use registered insecticide; keep plants well watered; use reflective mulch |
| Bean | Young leaves curled, distorted, and yellow; clusters of tiny insects on leaves and stems | Aphids | Use registered insecticide |
| Beets | Small, circular spots with light centers and dark borders on leaves | Cercospora leaf spot (fungal disease) | Pick off and destroy affected leaves, fungicides are not warranted |
| Beets | Roots cracked; black areas on surface and inside root; plants stunted | Boron deficiency | Soil test; maintain pH between 6 and 7; apply solution of household borax if necessary (1 T household borax per 12 gal water per 100 ft row) |
| Beets | Leaf margins rolled upward; leaves brittle and puckered along veins; plants stunted | Viral disease | Control leafhoppers, which spread the disease; weed control |
| Beets | Misshapen roots | Overcrowding | Thin beets early |
| Beets | Misshapen roots | Lumpy soil | |
| Beets | Leaves riddled with tiny holes | Flea beetles | Treat early with registered insecticide |
| Beets | Irregular, tan blotches on leaves | Leafminers | Use registered insecticide; root is still edible |
| Beets | Root scarred or tunneled | Carrot weevil, carrot rustfly, or wireworms | Destroy infested plants; next year work in a soil insecticide at planting |
| Carrots | Brown spots on leaves; spots may appear on carrots also | Fungal or bacterial disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Carrots | Inner leaves yellowed, outer leaves reddish purple; roots stunted and bitter | Aster yellows (phytoplasma disease) | Remove affects plants; weed control; leafhopper control with registered insecticide |
| Carrots | Root tops green | Root tops exposed to sunlight | Cover exposed roots with soil or mulch |
| Carrots | Roots misshapen | Overcrowding | Thin carrots early |
| Carrots | Roots misshapen | Lumpy soil | |
| Carrots | Roots misshapen | Root knot (nematode problem) | Check roots for galls; rotate; grow cover crops antagonistic to nematodes before replanting crop; incorporate organic matter into soil to stimulate microbial activity antagonistic to nematodes; plant French marigolds to infested area for one year and incorporate into soil to reduce nematode populations; use resistant varieties if available |
| Carrots | Plants stunted and yellowed; roots misshapen; small knots on fibrous roots | Root knot (nematode problem) | Check roots for galls; rotate; grow cover crops antagonistic to nematodes before replanting crop; incorporate organic matter into soil to stimulate microbial activity antagonistic to nematodes; plant French marigolds to infested area for one year and incorporate into soil to reduce nematode populations; use resistant varieties if available |
| Carrots | Tiny holes on leaves | Flea beetles | Use registered insecticide |
| Carrots | Light brown blotches or tunnels in leaves | Leafminers | Use registered insecticide |
| Celery | Stalks tough and bitter | High temperatures | |
| Celery | Stalks tough and bitter | Dry soil | Celery requires high moisture |
| Celery | Stalks tough and bitter | Poor fertility | Soil test |
| Celery | Stalks tough and bitter | Overmaturity | Harvest when tender |
| Celery | Plants stunted and yellowed; stalks twisted and brittle | Aster yellows (phytoplasma disease) | Remove affects plants; weed control; leafhopper control with registered insecticide |
| Celery | Leaves curled and petioles twisted; small, sunken, light brown, elliptical lesions on stalks; pale green leaves and stalks; slimy, dark rot of celery hearts may be present | Anthracnose (fungal disease) | Avoid overhead irrigation; avoid planting celery near garlic or strawberries; rotate; plant less susceptible varieties, e.g. Meringo, Hadrian, Geronimo, Balada |
| Celery | Plants wilted; soft, watery rot on leaves and stalks; heart of plant may be black | Fungal or bacterial crown rot | No adequate controls; rotate and remove old plant debris |
| Celery | Plants wilted; soft, watery rot on leaves and stalks; heart of plant may be black | Black heart (due to calcium deficiency) | Calcium deficiency results from uneven water supply or improper pH; water during dry periods; soil test; maintain soil pH between 6.5 and 8 |
| Celery | Brown or gray spots on leaves and stalks | Fungal or bacterial disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Cole Crops | Cracking of cabbage heads | Excess water taken up by the plant causes head to burst | Harvest heads as soon as mature |
| Cole Crops | Cracking of cabbage heads | ||
| Cole Crops | Poor heading | Overcrowding | Thin plants early |
| Cole Crops | Poor heading | Dry soil | Supply water |
| Cole Crops | Poor heading | High temperatures | |
| Cole Crops | Poor heading | Poor fertility or improper soil pH | Soil test |
| Cole Crops | Poor heading | Root knot (nematode problem) | Check roots for galls; rotate; grow cover crops antagonistic to nematodes before replanting crop; incorporate organic matter into soil to stimulate microbial activity antagonistic to nematodes; plant French marigolds to infested area for one year and incorporate into soil to reduce nematode populations; use resistant varieties if available |
| Cole Crops | Poor heading | Clubroot (fungal disease) | Check roots for large, spindle-shaped swellings (larger than root knot galls); rotate cole crops out of affected area for 7 years |
| Cole Crops | Poor heading | Root rot (fungal or oomycete disease) | Rotate; remove old plant debris; plant in well-drained soil |
| Cole Crops | Discolored cauliflower heads | Exposure to sun | Tie leaves over head when heads form |
| Cole Crops | Brown spots on leaves | Fungal, bacterial or oomycete disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Cole Crops | Plants wilt and turn yellow; roots have large, spindle-shaped swellings (not to be confused with smaller root knots) | Clubroot (fungal disease) | 7-year rotation |
| Cole Crops | Plants wilt and turn yellow; roots are discolored and poorly developed; roots may be hard and brittle | Blackleg (fungal disease) | Use western-grown, hot-water-treated seed; rotate; remove old plant debris |
| Cole Crops | Plants wilt and turn yellow; roots are discolored and poorly developed; roots may be hard and brittle | Cabbage maggots | Work in a registered soil insecticide at planting time |
| Cole Crops | Plants stunted and yellowed (esp. cabbage), roots not discolored | Dry soil | Supply water |
| Cole Crops | Plants stunted and yellowed (esp. cabbage), roots not discolored | Poor fertility or improper soil pH | Soil test |
| Cole Crops | Plants stunted and yellowed (esp. cabbage), roots not discolored | Fusarium yellows (fungal disease) | Use resistant varieties; rotate |
| Cole Crops | Plants stunted and yellowed (esp. cabbage), roots not discolored | Cabbage maggots | Work in a registered soil insecticide at planting time |
| Cole Crops | Heads soft and rotted | Soft rot of broccoli (bacterial disease) | Grow broccoli varieties that shed water (conical head) |
| Cole Crops | Heads soft and rotted | Bottom rot of cabbage (fungal disease) | Rotate; plant in well-drained soil |
| Cole Crops | Rough, brown, raised areas on undersurface of leaves | Oedema, physiological problem due to uneven water supply | Water during dry periods |
| Cole Crops | Leaves riddled with tiny holes | Flea beetles | Use registered insecticide |
| Cole Crops | Leaves chewed | Imported cabbage worm, cabbage looper, diamondback moth, cross-striped cabbage worm, or flea beetle | Submit insect for identification; use registered insecticide for specific insect (see PMG) |
| Cole Crops | Some leaves curled and yellowed; clusters of small gray or green insects on affected plant parts | Aphids | Use registered insecticide |
| Corn | Ears not completely filled with kernels | Poor pollination | Plant in blocks of at least 3-4 short rows instead of 1 long row; hand pollinate |
| Corn | Ears not completely filled with kernels | Birds | Put paper bag over ear after pollination |
| Corn | Ears not completely filled with kernels | Western corn rootworm | Rotate |
| Corn | White smooth or black, oily galls on stalk, leaves, ears, or tassels | Smut (fungal disease) | Cut off galls before they turn black; remove old plant debris; use tolerant varieties |
| Corn | Brown lesions on stalks near joints; stalks rotted inside; kernels pink or brown and moldy | Fungal stalk and ear rot (any of several) | No adequate controls; remove old plant debris |
| Corn | Plants wilted and stunted; long, irregular brown streaks on leaves; brown cavities in stalks near soil line | Bacterial wilt | Control flea beetles and cucumber beetles; remove affected plants; use tolerant varieties |
| Corn | Yellowish or tan elliptical spots, initially on lower leaves | Fungal leaf spot (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Corn | Plants stunted with yellow and green stripe or mosaic pattern, older leaves pale yellow | Maize dwarf mosaic (viral disease) | Weed control, esp. Johnsongrass; aphid control; destroy affected plants; do not handle healthy plants after handling affected ones |
| Corn | Small pustules containing rust-colored, powdery substance on leaves | Rust (fungal disease) | Use resistant varieties; remove old plant debris |
| Corn | Plants fall down after rain | Western corn rootworm | Rotate |
| Corn | Numerous tiny brown spots on leaves | Fungal leaf spot (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Corn | Leaves reddish on margins | Phosphorus deficiency | Soil test |
| Corn | Leaves reddish on margins | Viral disease | Weed control before corn emerges; aphid control; remove affected plants |
| Corn | Distorted leaf or stalk: leaves may fail to unfurl or stalk may be bent | Herbicide injury (growth regulator-type herbicide) | Follow label rates and precautions when applying herbicides |
| Corn | Feeding on the tip of the ear | Corn earworm | Apply registered insecticide during silking to prevent infestation |
| Corn | Young plants chewed off at ground level | Cutworms | Use registered insecticide |
| Cucurbits | No fruit produced | Poor pollination | Be patient; male and female flowers are not produced at the same time at first; bee activity may be low due to cool weather or use of insecticides; spray insecticides in late afternoon when pollinators are not active |
| Cucurbits | Misshapen or bitter fruit | Poor pollination | See above for no fruit produced |
| Cucurbits | Misshapen or bitter fruit | Dry soil | Supply water |
| Cucurbits | Misshapen or bitter fruit | Poor soil fertility or improper soil pH | Soil test |
| Cucurbits | Watersoaked, sunken, brown or black spot at blossom end of fruit only | Calcium deficiency, usually caused by uneven soil moisture and poor supply of calcium to fruit during early development | Water during dry periods; apply calcium foliar spray |
| Cucurbits | Watersoaked, sunken, brown or black spots on fruit not restricted to blossom end | Fungal, bacterial or oomycete fruit rot (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Cucurbits | Wilted plants | Dry soil | Supply water |
| Cucurbits | Wilted plants | Bacterial wilt | Control cucumber beetles |
| Cucurbits | Wilted plants | Root rot (fungal or oomycete disease) | Improve drainage; rotate; remove old plant debris |
| Cucurbits | Wilted plants | Fusarium wilt (fungal disease) | Use tolerant varieties if available; rotate |
| Cucurbits | Wilted plants | Root knot (nematode problem) | Check roots for galls; rotate; grow cover crops antagonistic to nematodes before replanting crop; incorporate organic matter into soil to stimulate microbial activity antagonistic to nematodes; plant French marigolds to infested area for one year and incorporate into soil to reduce nematode populations; use resistant varieties if available |
| Cucurbits | Wilted plants | Squash vine borer | Destroy affected vine; use registered insecticide |
| Cucurbits | Wilted plants; if a cut stem piece is propped up in a glass of water so that the cut end remains suspended in the water (not touching bottom), a white, milky substance streams out within 15 minutes (make sure what you see is not debris or soil) | Bacterial wilt | Control cucumber beetles; remove affected plants |
| Cucurbits | Circular or irregular brown spots on leaves and/or fruit | Fungal, bacterial or oomycete disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Cucurbits | White, powdery growth on leaves; may be on both leaf surfaces | Powdery mildew (fungal disease) | Use resistant varieties; use registered fungicide; remove old plant debris |
| Cucurbits | Yellow or brown, angular spots on upper leaf surfaces; grayish fuzzy growth on underside of spots (visible with hand lens) | Downy mildew (oomycete disease) | Use resistant varieties; use registered fungicide; remove old plant debris |
| Cucurbits | Yellow and green mottled pattern on leaves; leaves have strapped appearance i.e. abnormally narrow with leaf veins extending beyond leaf margins so that leaves appear feathery | Viral disease | Weed control before plants emerge; aphid control; remove affected plants |
| Cucurbits | Yellow and green mottled pattern on leaves; leaves have strapped appearance i.e. abnormally narrow with leaf veins extending beyond leaf margins so that leaves appear feathery | Herbicide injury (growth regulator-type herbicide) | If lawn herbicides are used, apply after wind has died down and do not apply in heat of day; avoid using mulch from herbicide-treated fields, manure from animals fed on herbicide-treated fields or grass clippings from herbicide-treated lawns |
| Cucurbits | Holes chewed in leaves and stems; yellow-green beetles with black stripes or spots | Cucumber beetles | Use registered insecticide |
| Cucurbits | Squash and pumpkin leaves wilt, eventually become black and crisp; dark gray bugs 1/2 inch long present | Squash bug | Use registered insecticide |
| Garlic | Leaves turn yellow, wilt and die back; tiny, black, spherical structures the size of a pin head appear on surface of bulb | White rot (fungal disease) | Avoid moving infested soil to other areas on shoes, tools, etc., as sclerotia (the tiny black structures) overwinter and are long-lived in soil; carefully remove affected plants and the soil around them and discard; rotate with non-allium crops; use only healthy garlic for planting |
| Eggplant | Blossoms drop; no fruit develops | Poor pollination due to unfavorable temperatures | Be patient, fruit will set when temperatures become more favorable |
| Eggplant | Plants wilt; bottom leaves may turn yellow | Dry soil | Supply water |
| Eggplant | Plants wilt; bottom leaves may turn yellow | Verticillium wilt or Fusarium wilt (fungal diseases) or bacterial wilt | Submit sample for laboratory diagnosis; rotate; remove old plant debris; do not plant tomatoes, strawberries, potatoes, or brambles in the same area; use resistant varieties for specific disease |
| Eggplant | Plants wilt; bottom leaves may turn yellow | Waterlogged soil | Improve drainage |
| Eggplant | Plants wilt; bottom leaves may turn yellow | Root knot (nematode problem) | Check roots for galls; rotate; grow cover crops antagonistic to nematodes before replanting crop; incorporate organic matter into soil to stimulate microbial activity antagonistic to nematodes; plant French marigolds to infested area for one year and incorporate into soil to reduce nematode populations; use resistant varieties if available |
| Eggplant | Plants wilt; bottom leaves may turn yellow, brown discoloration inside stem | Verticillium wilt or Fusarium wilt (fungal diseases) or bacterial wilt | Submit sample for laboratory diagnosis; rotate; remove old plant debris; do not plant tomatoes, strawberries, potatoes, or brambles in the same area; use resistant varieties for specific disease |
| Eggplant | Plants wilt; bottom leaves may turn yellow, brown discoloration inside stem | Waterlogged soil | Improve drainage |
| Eggplant | Plants wilt; bottom leaves may turn yellow, brown discoloration inside stem | Walnut wilt | Rule out vascular wilt disease with laboratory diagnosis; do not plant garden near walnut or butternut trees; sever roots between garden and tree and put in barrier |
| Eggplant | Circular or irregular brown spots on leaves and/or fruit | Fungal or bacterial disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Eggplant | Leaves riddled with tiny holes | Flea beetles | Use registered insecticide |
| Lettuce | Bolting; may taste bitter | Weather too hot | Lettuce is a cool season crop; plant early or late |
| Lettuce | Sunken, water-soaked spots appear on lower leaves, which turn brown and slimy; heads turn brown | Rhizoctonia bottom rot (fungal disease) | Rotate; remove old plant debris; plant in well-drained area |
| Lettuce | Sunken, water-soaked spots appear on lower leaves, which turn brown and slimy; heads turn brown | Sclerotinia drop (fungal disease) | Rotate; remove old plant debris; plant in well-drained area |
| Lettuce | Sunken, water-soaked spots appear on lower leaves, which turn brown and slimy; head turns brown and slimy; hard, black, pea-sized pellets found in mold between dead leaves | Sclerotinia drop (fungal disease) | Rotate; remove old plant debris; plant in well-drained area |
| Lettuce | Stem and lower leaves rotted; dense, fuzzy gray mold on affected areas | Botrytis gray mold (fungal disease) | Rotate; remove old plant debris; plant in well-drained area |
| Lettuce | Yellow or brown, angular blotches on upper leaf surfaces; white, fuzzy mold on underside of blotches (visible with hand lens) | Downy mildew (oomycete disease) | Rotate; use registered fungicide; use resistant varieties |
| Lettuce | Plants stunted; yellowed; youngest leaves curled; head soft | Aster yellows (phytoplasma disease) | Remove affected plants; weed control; insect control |
| Lettuce | Plants stunted; yellowed; youngest leaves curled; head soft | Virus | Remove affected plants; weed control; insect control |
| Lettuce | Plants stunted; yellowed; youngest leaves curled; head soft | Nutrient deficiency or improper soil pH | Soil test |
| Lettuce | Leaf veins and area adjacent to veins turns light yellow causing a “big vein” effect | Big vein (viroid disease) | Plant in well-drained soil: viroid (virus-like particle) is spread by a soil fungus; remove affected plants; rotate out of area for 10 years |
| Onion | Watersoaked spots appear on the leaves and rapidly turn brown; spots become purplish with a dark margin and surrounded with a yellow halo; spots become covered with brown, dusty mold in moist weather | Purple blotch (fungal disease) | Use registered fungicide |
| Onion | Numerous small white flecks on leaves; leaves die from tips back and turn brown | Botrytis blast (fungal disease) | Use registered fungicide |
| Onion | Numerous small white flecks on leaves; leaves die from tips back and turn brown | Downy mildew (oomycete disease) | Use registered fungicide |
| Onion | Numerous small white flecks on leaves; leaves die from tips back and turn brown | Onion thrips (insect) | Use registered insecticide; keep plants well watered; use reflective mulch |
| Onion | White flecks form on leaves and expand into elongated leaf lesions; white to purplish mold (visible with hand lens), develops on spots during moist weather; leaves drop and dry up | Downy mildew (oomycete disease) | Rotate; Use registered fungicide |
| Onion | Leaves yellow and die back from tips; bulbs are soft and rotted | Fungal or bacterial bulb rot (any of several) | Rotate; remove old plant debris; plant in well-drained soil |
| Onion | Dark green or black smudge up to 1 inch in diameter on bulb or neck; dark smudge is covered with stiff bristles (visible with hand lens) | Smudge (fungal disease) | Rotate; remove old plant debris; plant in well-drained soil |
| Onion | Plants grow slowly, wilt, and die; white maggots inside bulb | Onion maggots | Work registered insecticide into soil; destroy infested onions |
| Onion | White streaks or blotches on leaves | Onion thrips | Use registered insecticide; keep plants well watered; use reflective mulch |
| Peas | Plants stop producing pods; leaves turn yellow, then brown and die | Hot weather | Peas are cool-season vegetables; plant early in spring; plant heat-tolerant varieties |
| Peas | Plants stop producing pods; leaves turn yellow, then brown and die | Root rot (any of several fungi or oomycetes) | Rotate; plant in well-drained soil; remove old plant debris |
| Peas | Plants stop producing pods; leaves turn yellow, then brown and die | Fusarium wilt (fungal disease) | Use resistant varieties; rotate; remove old plant debris |
| Peas | Plants stunted; lower leaves yellowed; internal stem tissue discolored brown | Fusarium wilt (fungal disease) | Use resistant varieties; rotate; remove old plant debris |
| Peas | Plants stunted; lower leaves yellowed; internal stem tissue discolored brown | Waterlogged soil | Improve drainage |
| Peas | White, powdery mold develops on upper and then lower surfaces of leaves; leaves and pods may be distorted | Powdery mildew (fungal disease) | Rotate; remove old plant debris |
| Peas | Brown or white spots on leaves, pods, and/or stems | Fungal or bacterial disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Peas | Brown or white spots on leaves, pods, and/or stems | Thrips, aphids, or leafhoppers | Submit insect for identification; use registered insecticide for specific insect (see PMG) |
| Peas | Yellowish areas on leaves; blister-like ridges on undersides of leaves and on pods; pod distortion | Pea enation mosaic virus | Use resistant varieties; weed control; insect control; remove affected plants |
| Peas | Light-colored leaf veins; rosetting of shoot tips; plants stunted with poor pod set | Pea stunt virus | Weed control; insect control; remove affected plants |
| Peas | Yellow and green mottle or mosaic pattern on leaves; plants stunted | Viral disease (any of several) | Use resistant varieties if available; weed control; insect control |
| Peppers | Large, sunken, tan, watersoaked spot develops on blossom end of fruit; spot turns black and mold may grow on surface | Blossom end rot, caused by calcium deficiency to developing fruits | Calcium deficiency is a problem when developing fruits receive uneven moisture; supply water during dry periods; mulch plants |
| Peppers | Thin, wrinkled tan areas develop on fruit and become white and papery | Sunscald | Control leaf diseases with registered pesticides to prevent leaf drop, which exposes fruit to sun |
| Peppers | Brown frass-filled tunnels in fruit | European corn borer | Use registered insecticide |
| Peppers | Dark brown, sunken spots develop on fruit (not restricted to blossom end) and leaves | Fungal or bacterial disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Peppers | Small tan to dark brown, watersoaked spots develop on leaves; small brown, dry raised spots appear on fruit | Bacterial spot | Use fixed copper bactericide (e.g. Kocide) if available; use bleach-treated or western grown, hot-water-treated seed; avoid overhead watering |
| Peppers | Tiny, brown specks with pale white haloes develop on fruit; fruit may be distorted around specks | Stink bug injury | Use registered insecticide |
| Peppers | Brown spots on leaves | Fungal or bacterial disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Peppers | Plants stunted; leaves with yellow and green mottle; leaves curled; fruit misshapen with brown streaks, rings or yellow, green, and red mottle | Viral disease (any of several) | Use resistant varieties if available; weed control; insect control; remove old plant debris |
| Peppers | Plants wilted; dark brown canker at base of stem | Fungal or bacterial disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Peppers | Plants wilted; dark brown canker at base of stem; small, hard, brown pellets form on soil and rotted plant tissue | Southern blight (fungal disease) | Rotate; remove old plant debris |
| Peppers | Plants wilt; lower leaves may turn yellow | Fungal or bacterial vascular wilt disease | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Peppers | Plants wilt; lower leaves may turn yellow | Dry soil | Supply water |
| Peppers | Plants wilt; lower leaves may turn yellow | Waterlogged soil | Improve drainage |
| Peppers | Plants wilt; lower leaves may turn yellow | Root rot (fungal or oomycete disease) | Rotate; remove old plant debris; plant in well-drained soil |
| Potato | Potato tuber is green | Exposure to sun | Mound soil up around plants; do not eat green parts of potatoes |
| Potato | Tubers with tunneling white worms present | Potato tuberworm | Provide adequate soil coverage when hilling potatoes; harvest as soon as crop is mature; destroy any culled potatoes as soon as possible; store tubers below 52F; screen storage area to prevent entry of moths |
| Potato | Brown spots on leaves and/or stems | Various fungal diseases | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Potato | Plants wilt; bottom leaves may turn yellow | Dry soil | Supply water |
| Potato | Plants wilt; bottom leaves may turn yellow | Vascular wilt (fungal or bacterial disease) | Rotate; remove old plant debris |
| Potato | Plants wilt; bottom leaves may turn yellow | Root rot (fungal or oomycete disease) | Rotate; remove old plant debris; plant in well-drained soil |
| Potato | Plants wilt; bottom leaves may turn yellow | Root knot (nematode problem) | Check roots for galls; rotate; grow cover crops antagonistic to nematodes before replanting crop; incorporate organic matter into soil to stimulate microbial activity antagonistic to nematodes; plant French marigolds to infested area for one year and incorporate into soil to reduce nematode populations; use resistant varieties if available |
| Potato | Plants wilt; bottom leaves may turn yellow | Waterlogged soil | Improve drainage |
| Potato | Plants wilt; dark brown or black canker at base of stem | Fungal or bacterial disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Potato | Brown, corky scabs or pits on tubers; plants do not wilt | Scab (bacterial disease) | Soil test; acidify soil with aluminum sulfate if necessary to maintain pH of 5.0-5.5; rotate out of area for 3-4 years; use tolerant varieties; use certified seed pieces |
| Potato | Plants stunted; leaves turn bronze to yellow color; plants wilt; tubers have raised, knotty areas | Root knot (nematode problem) | Check roots for galls; rotate; grow cover crops antagonistic to nematodes before replanting crop; incorporate organic matter into soil to stimulate microbial activity antagonistic to nematodes; plant French marigolds to infested area for one year and incorporate into soil to reduce nematode populations; use resistant varieties if available |
| Potato | Tubers show irregular white or brown cavities when cut open | Hollow heart, caused by plants growing too rapidly | Do not over-fertilize or plant too far apart |
| Potato | Shoot tips stunted, forming rosette; leaves turn yellow, then brown between veins; leaf margins curl upward; individual shoots may wilt; tubers show dark brown discolored ring internally when cut open | Ring rot (bacterial disease) | Submit sample for laboratory diagnosis; ring rot and brown rot are difficult to distinguish; discard infected tubers; plant certified seed pieces |
| Potato | Shoot tips stunted, forming rosette; leaves turn yellow, then brown between veins; leaf margins curl upward; individual shoots may wilt; tubers show dark brown discolored ring internally when cut open | Brown rot (bacterial disease) | Submit sample for laboratory diagnosis; ring rot and brown rot are difficult to distinguish; discard infected tubers; plant certified seed pieces |
| Potato | Irregular brown discoloration in tubers | Early frost | |
| Potato | Irregular brown discoloration in tubers | Drought | Supply water |
| Potato | Irregular brown discoloration in tubers | Viral disease (any of several) | Use resistant varieties if available; weed control; insect control |
| Potato | Leaves stippled with dark specks; have bronzed appearance and die starting with lowest leaves | Ozone injury | |
| Potato | Deformed tubers, e.g. dumbbell or other shapes | “Second growth” due to extremes in moisture and/or temperature | Maintain uniform moisture by watering and mulching |
| Potato | Tubers have slimy, smelly rot | Soft rot (bacterial disease) | Plant in well-drained soil; hill plants to encourage water runoff; wait until vines turn yellow and die to dig; store properly |
| Potato | Leaves roll upward, turn light green to yellow and leathery; plants stunted | Leaf roll (viral disease) | Plant certified seed pieces; insect control; weed control |
| Potato | Leaves roll upward, turn purple or yellow; plants stunted; aerial tubers form | Aster yellows (phytoplasma disease) | Leafhopper control; weed control |
| Potato | Tunnels in tubers | Wireworms | Use a registered soil insecticide at planting time |
| Potato | Leaves chewed; fat, red, humpbacked grubs or orange beetles with black stripes present | Colorado potato beetles | Hand pick beetles or use registered insecticide |
| Radish | Yellow spots develop on upper leaf surfaces and later turn brown with bluish-black lace-like markings; white mold develops on undersurface of spots (visible with hand lens); inner root tissue may be discolored | Downy mildew (oomycete disease) | Remove old plant debris; rotate |
| Radish | Purple to black spots develop on root surface; black discoloration extends inward in radial streaks; roots remain firm | Black root (fungal disease) | Plant in well-drained soil; rotate; remove old plant debris |
| Radish | Leaves riddled with tiny holes | Flea beetles | Use registered insecticide |
| Spinach | Bolting | Hot weather and long days | Spinach is a cool-season crop; plant in early spring |
| Spinach | Pale yellow spots appear on upper leaf surfaces; grayish purple mold develops on underside of spots (visible with hand lens); whole leaves may wither | Downy mildew (oomycete disease) | Fungicides are registered but usually not practical; remove affected leaves and old plant debris |
| Spinach | White, blister-like spots with a yellow halo appear on undersides of leaves; upper surfaces are pale green to yellow | White rust (oomycete disease) | Fungicides are registered but usually not practical; remove old plant debris; 3-year rotation |
| Spinach | Irregular tan blotches or tunnels appear on leaves; tunnels are translucent when held up to light | Leafminers | Use registered insecticide before leafminer eggs are laid |
| Sweet Potato | Large cracks in potato skin | Growth cracks, caused by moisture extremes | Supply water during dry periods |
| Sweet Potato | Brown or black spots on potato skin; discoloration extends beneath skin | Various fungal diseases | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Sweet Potato | Brown, irregular blotches on potato skin; discoloration does not extend beneath skin | Scurf (fungal disease) | 3-4 year rotation; use disease-free slips; eat infected potatoes soon since they will dry out rapidly |
| Tomato | Uniformly small (1/8”) chocolate brown spots or dark spots with tan centers develop on leaves from bottom of plant to top; spots sometimes form on stems but never on fruits; leaves shrivel | Septoria leaf spot (fungal disease) | Use registered fungicide; remove old plant debris |
| Tomato | Uniformly small (1/8”) chocolate brown spots or dark spots with tan centers develop on leaves from bottom of plant to top; spots sometimes form on stems but never on fruits; leaves shrivel | Bacterial spot | Not as common as Septoria leaf spot; use bleach-treated seed and fixed copper (e.g. Kocide) spray if available. |
| Tomato | Dark brown irregular spots with target rings and yellow haloes develop on leaves, stems, and fruit; spots on fruit are often at stem end and are sunken | Early blight (fungal disease) | Use resistant varieties; use registered fungicide; remove old plant debris |
| Tomato | Dark brown irregular spots with target rings and yellow haloes develop on leaves, stems, and fruit; spots on fruit are often at stem end and are sunken | Phoma rot (fungal disease) | Phoma rot is not as common as early blight; use registered fungicide; remove old plant debris |
| Tomato | Light tan spots on upper leaf surfaces; dense, olive green moldy growth on undersurface of spot | Gray leaf mold (fungal disease) | Mainly a greenhouse problem: provide adequate ventilation to avoid high humidity; fungicides used to control other diseases will control this disease in the garden |
| Tomato | Small (1/8”) chocolate brown spots on leaves and fruit; spots on fruit are raised and scabby | Bacterial spot | Use bleach-treated seed; avoid overhead watering; used fixed copper bactericide (e.g. Kocide) if available; remove old plant debris; rotate |
| Tomato | Very tiny, raised specks on fruit; no white haloes around spots | Bacterial speck | Same controls as for bacterial spot |
| Tomato | Very tiny raised specks surrounded by white haloes on fruit; plants wilt; center of stem appears discolored brown when cut longitudinally; marginal leaf scorch with a band of chlorosis inside the brown scorched edge | Bacterial canker | Difficult to diagnose without fruit spots; same controls as for bacterial spot and speck |
| Tomato | Brown spots on leaves that do not fit above descriptions | Various fungal leaf spots | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Tomato | Dark brown, leathery spot on blossom end of fruit only; mold may grow on spot | Blossom end rot, caused by calcium deficiency to developing fruits during dry periods; moldy growth is secondary on dead tissue | Calcium deficiency is a problem when fruits receive uneven moisture during early development; supply water; apply calcium foliar spray; mulch |
| Tomato | Dark brown, sunken spots on fruits | Various fungal fruit rots | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Tomato | General browning of tomato skin; brown speckling of walls between seed cavities apparent when fruit is cut open | Internal browning (viral disease) | Use resistant varieties (resistant to Tobacco Mosaic Virus); weed control; do not handle healthy plants after diseased ones; remove affected plants |
| Tomato | Extreme malformation and scarring of fruit during fruit formation | Catfacing, caused by cool weather or herbicide injury from growth regulator-type herbicide | If lawn herbicides are used, apply after wind has died down and do not apply in heat of day; avoid using mulch from herbicide-treated fields, manure from animals fed on herbicide-treated fields or grass clippings from herbicide-treated lawns |
| Tomato | Yellow-orange blotches that do not ripen at stem end of fruit or white, papery spot on side of fruit facing sun | Sunscald | Prevent foliar diseases that cause leaf drop and expose fruits to sun |
| Tomato | Leaves distorted with strapped or feathery look (leaves narrower than normal, tips stretched out into thin projection, veins very close together) | Herbicide injury (growth regulator-type herbicide) | If lawn herbicides are used, apply after wind has died down and do not apply in heat of day; avoid using mulch from herbicide-treated fields, manure from animals fed on herbicide-treated fields or grass clippings from herbicide-treated lawns |
| Tomato | Leaves distorted with strapped or feathery look (leaves narrower than normal, tips stretched out into thin projection, veins very close together) | Cucumber mosaic (viral disease) | It is impossible to distinguish Cucumber Mosaic Virus from herbicide injury based on symptoms alone; however, if samples comes during spring when lawn herbicides are being sprayed, strongly suspect herbicide injury; virus is controlled by removing affected plants, weed control and aphid control |
| Tomato | Leaves roll upward, feel leathery, but remain green; plants are not stunted | Excess water | Common physiological disorder after wet periods; varieties Big Boy, Floramerica, and Beefsteak are especially susceptible |
| Tomato | Plants wilted; bottom leaves may turn yellow; brown discoloration inside stem (in vascular ring) | Fungal or bacterial vascular wilt disease | Submit sample for laboratory analysis; resistant varieties are available for some vascular wilt diseases |
| Tomato | Plants wilted; bottom leaves may turn yellow; brown discoloration inside stem (in vascular ring) | Walnut wilt, caused by toxin from walnut tree | Rule out vascular wilt disease with laboratory diagnosis; do not plant tomatoes near walnut or butternut trees; sever roots bordering garden and place barrier between tree and garden |
| Tomato | Plants stunted, wilted, and yellowed; nodules on roots | Root knot (nematode problem) | Check roots for galls; rotate; grow cover crops antagonistic to nematodes before replanting crop; incorporate organic matter into soil to stimulate microbial activity antagonistic to nematodes; plant French marigolds to infested area for one year and incorporate into soil to reduce nematode populations; use resistant varieties if available |
| Tomato | Young plants cut off at ground level | Cutworms | Use cutworm collars or registered insecticide |
| Tomato | Young plants with many tiny holes in leaves | Flea beetles | Tomatoes will tolerate a lot of flea beetle damage if they are healthy; when necessary, use a registered insecticide |
| Tomato | Tiny, white-winged insects on undersides of leaves | Whiteflies | Yellow sticky boards (smeared with grease) will attract and trap adults or use registered insecticide |
Table 6-3: Tree Fruit & Nuts Diagnostic Key
| Crop | Symptoms | Possible Causes | Controls & Comments |
|---|---|---|---|
| Many | Premature fruit drop | Natural thinning | Many trees produce more fruit than they need and thin themselves naturally |
| Many | Premature fruit drop | Spring frost | Frost often kills developing fruits or buds |
| Many | Premature fruit drop | Poor pollination | Tree may require an appropriate pollinator tree nearby to pollinate it; be careful not to kill bees with insecticides |
| Many | Premature fruit drop | Environmental stress | Drought, cold, or hear can cause fruit to drop |
| Many | Premature fruit drop | Disease stress | See controls under specific diseases |
| Many | Premature fruit drop | Use of Sevin insecticide | Sevin causes some fruit thinning; do not misuse |
| Many | Premature fruit drop | Various insects | Submit insect for laboratory identification |
| Many | Poor fruit development (small number of fruit on tree) | Poor pollination | Tree may require an appropriate pollinator tree nearby to pollinate it; be careful not to kill bees with insecticides |
| Many | Poor fruit development (small number of fruit on tree) | Biennial bearing | Some apples and pears may produce a heavy crop one year and few fruits the following year |
| Many | Poor fruit development (small number of fruit on tree) | Improper pruning | Do not prune off fruit-bearing wood during the dormant season; consult pruning chapter for proper pruning timings |
| Many | Poor fruit development (small number of fruit on tree) | Frost injury | |
| Many | Fruits too small | Failure to prune or thin fruit | Peaches, nectarines, plums, and apples tend to produce many small fruits if not pruned; consult pruning chapter for proper instructions |
| Many | Fruits too small | Poor soil fertility | Soil test |
| Many | Fruit misshapen; “cat faces” | Tarnished plant bug | Follow spray schedule |
| Many | Many small twigs broken off | Squirrel damage | Squirrels prune twigs for nest-building and often prune more than they need |
| Many | Many small twigs broken off | Wind damage | |
| Many | Oozing sap on branches or trunk | Natural gummosis | Cherries, plums, apricots, and peaches naturally ooze sap |
| Many | Oozing sap on branches or trunk | Environmental stress | Drought or waterlogging can cause fruit trees to ooze sap excessively |
| Many | Oozing sap on branches or trunk | Mechanical injury | |
| Many | Oozing sap on branches or trunk | Disease or insect damage | See section on specific diseases and insects |
| Many | Oozing sap on branches or trunk | Shothole borer | Promote vigorous growth |
| Many | Large areas of split bark; no decay evident | Frost cracks | Frost can split tree trunks if sap in trunk expands; use tree-wrap to protect bark from sun to prevent extremes in temperature |
| Many | Large areas of split bark; no decay evident | Sunscald | Thin-barked trees, e.g. young ones, split when exposed to intense sunlight; use tree wrap or block sun with board on bright days |
| Many | Large areas of split bark; no decay evident | Mechanical injury, e.g. lawn mower | Dig up grass around trunk and replace with mulch to avoid mowing too closely to base of tree |
| Many | Large areas of split bark; no decay evident | Lightning injury | |
| Many | Large areas of split bark; decay evident in wood | Secondary decay of any of the wounds described above | No adequate controls; remove loose bark; water and fertilize tree when necessary |
| Many | Large areas of split bark; decay evident in wood | Fungal or bacterial (any of several) | Same as for secondary decay canker |
| Many | Gray or white powdery growth on leaves or flowers; leaves and fruit may be distorted | Powdery mildew (fungal disease) | Use registered fungicide |
| Many | Black, sooty growth on leaves, stems, and/or fruit | Sooty mold (fungus that grows on honeydew substance secreted by aphids and other insects) | Identify insect then control as warranted |
| Many | Brown dead areas on leaf margins | Leaf scorch, caused by insufficient transport of water to leaves | Water tree deeply during dry periods; scorch is usually caused by hot, dry weather, but root rot or other root problems can cause leaf scorch, as well. |
| Many | Brown dead areas on leaf margins | Cold injury | This would appear suddenly. Check weather history. |
| Many | Trees wilted/may have poor color | Dry soil | Water deeply during drought |
| Many | Trees wilted/may have poor color | Root rot (fungal disease) | Improve drainage |
| Many | Trees wilted/may have poor color | Root knot or root feeding nematodes | Submit soil sample for nematode assay |
| Many | Trees wilted/may have poor color | Various fungal, bacterial, or viral diseases | Submit for laboratory analysis |
| Many | Trees wilted/may have poor color | Waterlogged soil | Improve drainage |
| Many | Interveinal yellowing of leaves; no wilting | Nutrient or mineral deficiency; incorrect soil pH | Soil test |
| Many | Interveinal yellowing of leaves; no wilting | Waterlogged soil, resulting in poor transport of nutrients to leaves | Improve drainage |
| Many | Large, corky galls at base of tree and on roots | Crown gall (bacterial disease) | Some galls can be pruned out, but it is best to consult with a certified arborist; trees may live for many years in spite of galls; avoid wounding trees and choose rootstock that is resistant to crown gall |
| Many | Young leaves curled and distorted; clusters of insects on undersides of leaves | Aphids | Use registered insecticide; thoroughly cover undersides of leaves |
| Many | Silk tents in branch crotches in spring | Eastern tent caterpillar | Physically remove tents or use registered insecticide when caterpillars are small |
| Many | Silk tents on ends of branches in mid or late summer | Fall webworm | Same as for Eastern tent caterpillar |
| Many | Crescent-shaped scars on fruit; whitish legless grubs with brown heads present | Plum curculio | Use registered insecticide on a regular schedule |
| Many | Leaves with tiny white spots, often dirty with webbing | Spider mites | Use registered miticide |
| Many | Bark encrusted with tiny, slightly raised bumps; apples may have red spots with white centers | San Jose scale | Use a dormant oil spray or treat with registered insecticide when eggs are hatching; consult PMG for timing |
| Apple and Pear | Leaf spots with brown to yellowish- brown centers with dark brown border; spots on fruit are initially small and brown; progression of rot is slow and decayed tissue is firm; the fungus can also cause cankers on twigs and limbs | Frogeye leaf spot, also called black rot (fungal disease) | Follow apple/pear fungicide spray schedule; remove mummied fruit and prune out cankered wood back to healthy tissue; infection of the fruit can occur at any time and also post-harvest; fire blight infected limbs and freeze damaged limbs may develop large black rot fungal cankers |
| Apple and Pear | Initial leaf spots small, circular brown to blackish, later becoming irregularly shaped with a dark border (frogeye); when leaf petioles are infected leaves yellow and defoliation occurs; no branch/twig symptoms; no fruit infection on majority of cultivars | Alternaria blotch (fungal disease) | Follow apple/pear spray schedule; prune out diseased shoots and remove fallen leaves |
| Apple and Pear | Olive-brown velvety spots on leaves and young fruit; fruit spots develop into brown corky lesions and mature fruit is distorted | Scab (fungal disease) | Plant scab resistant cultivars; follow apple/pear spray schedule for susceptible cultivars |
| Apple and Pear | Bright yellow spots with orange or black centers on upper surface of leaves; cuplike pustules on lower leaf surface; greenish/yellowish spots on fruit; leaf infection results in defoliation | Cedar Apple Rust (fungal disease) | Plant cedar apple rust resistant cultivars; follow apple/pear spray schedule for susceptible cultivars; do not plant Eastern red cedar (Juniperus virginiana) trees, which are the alternate hosts of the fungus |
| Apple and Pear | Brown, roughly circular leaf spots not fitting above descriptions | Fungal leaf spot (any of several) | Submit sample for laboratory analysis |
| Apple and Pear | Brown, roughly circular leaf spots not fitting above descriptions | Chemical injury | Some fungicides can cause spotting on certain varieties of fruit trees |
| Apple and Pear | Sunken, light brown circular spots that grow rapidly and develop concentric rings of salmon colored specks (spore masses) on fruit; fruit mummies form and hang on tree; apple tastes bitter; cankered branches | Bitter rot (fungal disease) | Use apple/pear fungicide spray schedule; remove fruit mummies from tree and ground; prune out cankered branches back to healthy wood |
| Apple and Pear | Circular clusters of tiny black specks and small sooty smudges on fruit | Fly speck and sooty blotch (two fungal diseases that commonly occur together) | fruit is still edible; specks can be rubbed off; since this is a cosmetic problem fungicide sprays are not needed for the home garden; the apple/pear spray schedule can be used if the appearance of the fruit is critical |
| Apple and Pear | Unlike cedar apple rust this rust does not affect foliage; typically near the calyx end of fruit sunken, deformed lesions develop that are dark green to brown; lesions extend deep into fruit leading to fruit loss | Cedar-quince rust (fungal disease) | Use apple/pear spray schedule; do not plant Eastern red cedar (Juniperus virginiana) trees, which are the alternate hosts of the fungus |
| Apple and Pear | Small, necrotic, dry, brown lesions on fruit skin and/or flesh; taste of lesion is bitter; usually appears in storage | Bitter pit (physiological problem) | Often associated with certain cultivars and/or environmental and cultural conditions; very susceptible cultivars include Cortland, Honeycrisp, Cox’s Orange Pippin; since various causal factors background info may help pinpoint the cause(s) |
| Apple and Pear | Spots on fruit that do not fit above descriptions | Fungal disease | Submit sample for laboratory analysis |
| Apple and Pear | Spots on fruit that do not fit above descriptions | Various insects | Use apple/pear insecticide program |
| Apple and Pear | Bark on young branches is rough and pimply; tissue beneath bark has brown spots | Measles, believed to be a nutrient imbalance | Soil test |
| Apple and Pear | In springtime, leaves wilt, curl, and cling to twigs; shoot tip may be curved into “shepherd’s crook”; sunken, black or wine-colored cankers on young twigs, larger branches, or trunk. | Fire blight (bacterial disease) | Plant trees and rootstock resistant to fire blight; prune out affected branches on which bacteria overwinter; in late summer remove young suckers as they appear since they are very susceptible to fire blight; do not plant apples near pears which are highly susceptible |
| Apple and Pear | Tree breaks off at graft union during strong winds | Poorly constructed graft | Purchase young transplants from reliable dealer |
| Apple and Pear | Tree breaks off at graft union during strong winds | Virus infection at graft union | Submit soil sample for nematode analysis; some of these viruses are transmitted by nematodes in the soil |
| Apple and Pear | Pink-white worms bore into blossom end of apple; clusters of round, brown frass pellets inside fruit | Codling moth | Use registered insecticide on a regular schedule |
| Apple and Pear | Apples dimpled with faint brown areas in flesh | Apple maggot (“railroad streaks in the flesh worm”) | Use registered insecticide on a regular spray schedule |
| Stone fruits | Initial symptoms are small, angular, gray leaf spots that later turn purple, then necrotic; shothole may also occur; leaves with multiple spots may drop and susceptible cultivars may defoliate; the bacterium also causes twig cankers/dieback and spots on fruit that are first visible several weeks after petal fall and can progress to cracking, gum exudation and fruit loss | Bacterial spot (bacterial disease) | Plant cultivars of stone fruit that are resistant to bacterial spot. Once symptoms are observed it is too late to control the bacterium. Prune out diseased twigs back to healthy wood, since the bacterium overwinters in twig cankers. Ensuring adequate fertility is also important in avoiding this disease, since weakened trees are more susceptible. Appropriately labeled copper fungicides can be used for suppression, but phytotoxicity is a risk if applied during the growing season. Apply at leaf fall in the autumn and/or before bud break. |
| Stone fruits | Initial symptoms are small, angular, gray leaf spots that later turn purple, then necrotic; shothole may also occur; leaves with multiple spots may drop and susceptible cultivars may defoliate; the bacterium also causes twig cankers/dieback and spots on fruit that are first visible several weeks after petal fall and can progress to cracking, gum exudation and fruit loss | Nitrogen deficiency OR a captan + oil spray can cause similar symptoms on leaves and defoliation, so accurate diagnosis is important | |
| Stone fruits | Small, circular, olive-green spots on young fruit; spots eventually turn brown and velvety; similar spots on leaves | Scab (fungal disease) | Use spray schedule for stone fruits; prune properly so that sprays penetrate the canopy |
| Stone fruits | Purple spots appear on upper surfaces of cherry leaves; leaf spots drop out leaving holes, and turn yellow; fruit may also be spotted | Cherry leaf spot (fungal disease) | Plant resistant cherry cultivars; use fungicidal spray program recommended for stone fruits |
| Stone fruits | Peach or nectarine leaves puckered, thickened, and curled from the time they first appear in the spring; leaves red or orange at first but turn yellow; shoots swollen and stunted | Peach leaf curl (fungal disease on peaches and nectarines) | Follow stone fruit spray schedule for peach and nectarine |
| Stone fruits | Blossoms and young twigs wilt and decay during bloom; sunken cankers with gummy ooze develop on twigs; circular brown spots which develop tufts of gray spores during moist weather form on fruit; rot may cover large portion of fruit | Brown rot (fungal disease common to all stone fruit) | Follow stone fruit fungicidal spray schedule; remove all diseased fruit from tree and on ground: decayed fruit turns into a “mummy” on which the fungus overwinters |
| Stone fruits | Sunken cankers on twigs, larger branches and/or trunk; leaves above canker wilt | Fungal or bacterial disease (any of several) | Submit branch samples that include the junction of healthy and dead tissue for laboratory analysis. Prune out dead wood on branches back to healthy tissue. There are no management recommendations for cankers on the trunk. |
| Stone fruits | Swellings that split the bark appear on plum or cherry branches and later turn coal-black; leaves may wilt above swellings | Black knot (fungal disease of plum and cherry only) | Follow spray schedule. Prune out affected twigs at least 4” below knots; if practical remove wild plum and wild cherry trees in the area, since they provide inoculum for initial infections. There are plum cultivars with resistance to this disease and these are recommended for new plantings. |
| Stone fruits | Shoot tips stunted; leaves yellow and curled upward; severe defoliation; trees tend to break off near ground in strong winds; base of trunk may be swelled | Stem pitting (viral disease; primarily of peach) | Submit soil sample for nematode analysis; nematodes can transmit the virus or it can come in on the transplants; remove and destroy the affected trees |
| Stone fruits | Shoot tips stunted; leaves yellow and curled upward; severe defoliation; trees tend to break off near ground in strong winds; base of trunk may be swelled | Nutrient deficiency | Tissue analysis to check for nitrogen deficiency (private laboratory), soil test and analyze fertilization regime. |
| Stone fruits | Shoot tips stunted; leaves yellow and curled upward; severe defoliation; trees tend to break off near ground in strong winds; base of trunk may be swelled | Various other viral diseases | Purchase certified virus-free plants; remove any virus-infected plants; manage weeds. There are no control options for viruses other than avoidance and removal of diseased plants. Since different viruses are spread by different mechanisms, identifying the specific virus can help determine appropriate control tactics. Testing for specific viruses is limited, so it is advisable to start with submission of a digital sample. |
| Stone fruits | New growth at tip of twig wilts and dies; resin at tip of twig; maturing fruit may contain 1/2 long pinkish worms | Oriental fruit moth | Use registered insecticide to prevent damage; peach will tolerate a lot of natural pruning by this insect, so insecticides may not be necessary |
| Stone fruits | Gum oozes from holes at base of trunk or lower branch crotches; sawdust may be evident | Peach tree borers | Use registered insecticide on bark only |
| Stone fruits | Many small round holes in twigs and branches | Shothole borer | Remove and destroy all dead or dying wood; use registered insecticide to protect healthy trees |
| Stone fruits | Tiny white, flat insects encrusting bark | White peach scale | Use a dormant oil or treat with registered insecticide; refer to PMG for application timings |
| Pecan | Small, olive-colored spots on twigs and undersides of leaves; tiny black dots on shucks enlarge to form black lesions; nuts drop prematurely | Scab (fungal disease) | Plant scab-resistant cultivars of pecan. Raking and removing fallen leaves and nuts will reduce fungal inoculum for next season infection period. |
| Pecan | Leaf spots; no spots on nuts | Fungal disease (any of several) | Rake and remove fallen leaves to reduce inoculum available for future infections. Submit sample for laboratory analysis. There are limited fungicide controls for pecans in the home garden. |
| Pecan | Poor nut fill and/or premature nut drop | “Pops” – often a result of poor pollination | Poor pollination can result from stressful environmental conditions, such as drought, during nut development. Plant at least two different cultivars, spaced approximately 60 to 80 feet apart, to increase chances of good pollination. Another abiotic problem that can cause poor nut fill in pecans is called "water stage fruit split". Cultivars vary in susceptibility to this problem. Maintaining good soil moisture during the last two weeks prior to nuts reaching full size can help to prevent the problem. Other causes of premature nut drop include nutrient deficiency, which may be due to a soil problem, inadequate fertilization, inadequate water, crowded trees, etc. Also, bearing pecan trees require fertilizer. Soil samples can be submitted to check for soil nutrient deficiencies. In general, Virginia does not provide an optimum climate for pecan production. |
| Pecan | Nutmeats (kernels) have brown or black blotches and may be distorted | Feeding punctures (several species of plant bugs and stink bugs) | Remove any nearby weeds; treat with insecticide if insects found |
| Pecan | Small, cream-colored worms in immature nuts or in the green shucks after shells have hardened | Hickory shuckworm | Clean up and destroy fallen nuts to eliminate overwintering larvae |
| Walnut | See section on general problems. Most walnut diseases are difficult to diagnose without consulting a diagnostic laboratory. |
Table 6-4: Small Fruits Diagnostic Key
| Crop | Symptoms | Possible Causes | Controls & Comments |
|---|---|---|---|
| Many | Grayish or white moldy growth on leaves | Powdery mildew (fungal disease) | Follow appropriate pesticide spray schedule. |
| Many | Galls at base of plant, on roots, or on canes; plants stunted | Crown gall (bacterial disease) | Avoid wounding plants, which predispose plants to infection by this bacterium. Purchase disease-free plant material. Prune out galled tissue back to healthy tissue, if possible; there are no chemical controls for home growers. |
| Many | Plants wilt; leaves may turn yellow | Dry soil | Supply water |
| Many | Plants wilt; leaves may turn yellow | Waterlogged soil | Plant in well-drained area |
| Many | Plants wilt; leaves may turn yellow | Wilting can be caused by various abiotic and biotic (disease) problems. | Submit a plant and soil sample for diagnosis. |
| Many | Green and yellow mosaic or mottle pattern on leaves; plants may be stunted | Viral disease (any of several) | Purchase certified virus-free plants; remove any virus-infected plants to avoid spread; manage weeds, which may harbor virus inoculum. There are no control options for viruses other than avoidance and removal of diseased plants. Since viruses are spread by different mechanisms, both abiotic and biotic, identifying the specific virus can be helpful. However, testing for specific viruses is limited, so it is advisable to start with submission of a digital sample. |
| Many | Leaves rolled or tied together; small caterpillars feeding inside | Leafrollers | Follow spray schedule |
| Blueberry | Plants stunted and foliage off-color | Improper soil pH | Blueberries require acidic soil conditions; submit a soil sample for analysis |
| Blueberry | Plants stunted and foliage off-color | Nutrient deficiency | Submit a soil sample for analysis |
| Blueberry | Plants stunted and foliage off-color | Viral disease (any of several) | Purchase certified virus-free plants; remove any virus-infected plants to avoid spread; manage weeds, which may harbor virus inoculum. There are no control options for viruses other than avoidance and removal of diseased plants. Since viruses are spread by different mechanisms, both abiotic and biotic, identifying the specific virus can be helpful. However, testing for specific viruses is limited, so it is advisable to start with submission of a digital sample. |
| Blueberry | This fungus can infect shoots soon after bud break and also berries; white or pale-colored blueberries among normal berries are symptomatic of this disease; infected berries appear normal when green, then as they near maturity, turn cream and eventually tan or white; such berries become shriveled and hard and drop | Mummy berry (fungal disease) | Burying dropped diseased berries two inches in the soil or covering with 2” of fresh mulch will reduce inoculum for future infections. |
| Blueberry | The most common symptom of this disease is flagging and death the upper two to six inches of shoots during summer with leaves turning reddish and remaining attached to the twig; however, whole canes can also be affected | Phomopsis canker and twig dieback (fungal disease) | Prune out affected twigs or branches back to healthy tissue. Avoid planting in sites that are prone to spring frost, which can predispose plants to disease. Manage plants to discourage late season growth by irrigation and fertilizing according to recommendations. Refer to blueberry spray schedule for pesticide recommendation. |
| Blueberry | Branches die back; cankers may be evident | Fungal canker (any of several) | Submit sample for laboratory analysis |
| Blueberry | Branches die back; cankers may be evident | Winter injury | Damage is commonly observed in spring or early in the growing season |
| Blueberry | Ripening berries soft and mushy | Blueberry maggot | Use registered insecticide on a regular schedule |
| Brambles | Shoots are stunted and leaves turn yellow at bottom of plant, wilt and drop; overall plant wilts and dies back; stem may show dark blue color (not on red raspberry) at base; internal stem tissue may be discolored | Verticillium wilt (fungal disease) | Laboratory diagnosis needed; use certified disease-free plants; use resistant varieties, 3-4-year rotation |
| Brambles | Plants wilt, but yellowing and wilting does not begin at the bottom of the cane; stunting and foliar yellowing or marginal scorch; progressing to dieback | Dry soil | Supply water |
| Brambles | Plants wilt, but yellowing and wilting does not begin at the bottom of the cane; stunting and foliar yellowing or marginal scorch; progressing to dieback | Waterlogged soil | Improve drainage and/or modify irrigation |
| Brambles | Plants wilt, but yellowing and wilting does not begin at the bottom of the cane; stunting and foliar yellowing or marginal scorch; progressing to dieback | Phytophthora rot (oomycete disease) | Laboratory diagnosis needed; correct soil drainage; use a registered fungicide on adjacent non-symptomatic plants and replacement plants; remove affected plants |
| Brambles | Plants wilt, but yellowing and wilting does not begin at the bottom of the cane; stunting and foliar yellowing or marginal scorch; progressing to dieback | Root knot (nematode problem) | Check roots for knots; rotate |
| Brambles | Plants wilt, but yellowing and wilting does not begin at the bottom of the cane; stunting and foliar yellowing or marginal scorch; progressing to dieback | Planted too deeply (brambles do not tolerate deep planting) | Plant at proper depth |
| Brambles | Ripening berries covered with tufts of gray, green, white, or black moldy growth | Fungal fruit rot (any of several) | Harvest berries before they are over-ripe; cool immediately |
| Brambles | Small light grayish spots on canes that grow and develop dark border around a gray center; spots may be sunken; canes may crack; dieback; small spots on leaves are gray with purple margins; defoliation not typical | Anthracnose (fungal disease) | Submit sample for laboratory analysis; plant disease-free brambles; thin, prune and remove dead canes annually; prune out diseased canes; remove wild brambles in the vicinity; follow fungicide spray schedule |
| Brambles | Wilting; dieback; discoloration on canes; canes may be pimply, cracked or brittle | Fungal cane canker (any of several) | Submit sample for laboratory analysis; thin, prune and remove dead canes annually; prune out cankered canes |
| Brambles | Leaves curl downward; leaves smaller than usual; internodes shorter than normal | Leaf curl (viral disease) | Plant certified virus-free stock; remove affected canes; if more than 20% of canes are affected, remove entire planting; control aphids with registered insecticide; remove nearby wild brambles |
| Brambles | Leaves curl downward; leaves smaller than usual; internodes shorter than normal | Aphids or blackberry psyllid | Look for clusters of small gray insects on undersides of leaves; control with registered insecticide |
| Brambles | Leaves curl downward; leaves smaller than usual; internodes shorter than normal | Herbicide injury | Obtain background information on herbicides used in the vicinity |
| Brambles | Only on red or purple raspberry: yellow spore masses on fall fruit; powdery, yellow spore masses on undersides of leaves; leaves may drop | Late leaf rust (fungal disease) | Plant disease-free plants; plant resistant red and purple raspberry; promote foliar drying by cleaning out dead canes and manage weeds; remove infected canes post-season |
| Brambles | On black raspberry, blackberry and purple raspberry: In spring leaves on new shoots are stunted, deformed and off-color and shoots are spindly and many; blisters form on lower leaf surfaces and develop bright orange, powdery spores | Orange rust (fungal disease) | Plant disease-free plants and resistant cultivars; remove infected plants as soon as symptoms are observed; thin planting annually and manage weeds; remove nearby wild brambles |
| Brambles | Tip dies; rows of punctures around twig | Raspberry cane borer | Prune and destroy dead tips |
| Brambles | On blackberry: Leaf spots with a brown whitish center and a brown or purplish margin | Septoria leaf spot (fungal disease) | Space plants to allow proper air circulation and thin and remove dead canes annually; manage weeds; follow fungicide spray schedule for anthranose |
| Currant | Orange-brown blisters containing yellow spores appear on undersides of leaves; leaves turn yellow | White pine blister rust (fungal disease which has white pine as alternate host) | Use resistant varieties (Viking and Red Dutch); plant certified disease-free stock |
| Currant | Stems die back; cankers with tiny black pimple-like structures appear on stems | Fungal dieback (any of several) | Prune out affected stems |
| Currant | Brown leaf spots with tiny black pimply structures | Septoria leaf spot (fungal disease) | Use registered fungicide |
| Currant | Brown leaf spots without tiny black pimply structures | Fungal leaf spot (any of several) | Submit sample for laboratory analysis |
| Grape | Brown spots with dark borders on leaves; grapes turn black, shrivel up like raisins; and remain attached to stem | Black rot (fungal disease) | Use grape fungicide spray schedule; remove mummified berries since fungus overwinters on them |
| Grape | Brown leaf spots | Fungal leaf spot (any of several) | Submit sample for laboratory analysis |
| Grape | Small yellow spots appear on upper leaf surfaces; white cottony growth forms on undersides of spots (do not confuse white leaf hairs of some varieties with fungus) | Downy mildew (fungal disease) | Use grape fungicide spray schedule; Concord grape is resistant |
| Grape | Fruit rot not resembling black rot | Fungal fruit rot (any of several) | Submit sample for laboratory analysis |
| Grape | Canes die back; dark lesions on canes; tiny black lesions on leaves | Fungal dieback (most common one is Phomopsis dieback) | Prune well below cankers; use grape fungicide spray schedule |
| Grape | Leaf resembles a fan: main veins are drawn together and teeth along margins are elongated; plants stunted | Fan leaf (viral disease) | Purchase certified virus-free stock; submit soil sample for nematode analysis; remove affected plants |
| Grape | Leaf resembles a fan: main veins are drawn together and teeth along margins are elongated; plants stunted | Herbicide injury | Fan leaf and herbicide injury symptoms are identical: if symptoms occur in spring when lawn herbicides are being applied, herbicide injury is a good bet; do not apply lawn herbicides during hot or windy conditions |
| Grape | Small, green, seedless grapes are intermingled with ripe grapes in the cluster | Shot berry (physiological) | May be related to genetic, nutritional and environmental factors; no control |
| Grape | Grapes and/or leaves webbed together; some grapes collapsed | Grape berry moth | Use registered insecticide on regular schedule |
| Grape | Green or red irregular swellings on leaves, canes, or tendrils | Grape tomato gall (insect problem | Prune out and destroy heavily infested leaves and canes before the insects inside have matured |
| Grape | Small, rough galls the size of a small pea on the undersides of leaves; swellings on roots | Grape phylloxera (insect) | Use resistant varieties; no chemical controls |
| Strawberry | Small spots with white or tan centers and reddish-brown borders on leaves | Mycosphaerella leaf spot (fungal disease) | Use resistant varieties; use strawberry fungicide spray program |
| Strawberry | Purplish or brown spots on leaves not fitting above description | Fungal or bacterial leaf spot (any of several) | Submit sample for laboratory analysis |
| Strawberry | White or gray crusty material covering leaves, stems, and/or fruits | Slime mold (fungus) | Slime molds grow on plant surfaces during wet weather and disappear again in dry weather; no need for control |
| Strawberry | Gray, fuzzy mold on fruit, especially during wet periods and after frost | Gray mold (fungal disease) | Do not crowd plants; do not apply fertilizer in spring since dense foliage delays drying of berries after rains; follow strawberry spray schedule |
| Strawberry | Plants wilt; leaves may turn brown at margins; roots and/or crown appear discolored when cut open | Root and/or crown rot (fungal or oomycete disease) | Submit sample for laboratory diagnosis. If many plants show symptoms, replant in another area; plant in well-drained area; purchase disease-free transplants |
| Strawberry | Plants wilt; leaves may turn brown at margins; roots and/or crown appear discolored when cut open | Nematode injury | Submit soil sample for nematode analysis; soil fumigation |
| Strawberry | Fruit is hard and leathery with brown spots | Leather rot (oomycete disease) | Mulch with straw or bark to avoid fruit and soil contact; plant in well-drained location; rotate if high incidence. |
| Strawberry | Fruit is hard and leathery with brown spots | Environmental stress | Poor growing conditions can cause berries to become dry and hard |
| Strawberry | Fruit is soft with brown spots | Fungal fruit rot (any of several) | Laboratory diagnosis is needed. Mulch; Follow strawberry fungicide spray schedule if fungal disease is diagnosed |
| Strawberry | Malformed berries: look like several berries have grown together | Fasciation; a response to environmental conditions | Common in certain varieties in fall and spring |
| Strawberry | Berries seedy at tips | Insect injury | Use strawberry insecticide spray program |
| Strawberry | Berries seedy at tips | Mites | Use registered miticide |
| Strawberry | Berries seedy at tips | Frost injury | Protect plants from frost by mulching |
| Strawberry | Berries seedy at tips | Nutrient deficiency | Soil test |
| Strawberry | Flower buds droop, turn brown and may drop to ground | Strawberry bud weevil | Use registered insecticide during the bud stage |
| Strawberry | Small holes in leaves; poor growth | Strawberry rootworm | Rotate to new area |
Table 6-5- Ornamental Shrubs and Trees Diagnostic Key
| Crop | Symptoms | Possible Causes | Controls & Comments |
|---|---|---|---|
| Many | Many small twigs broken off | Squirrel damage | Squirrels prune twigs for nest-building and often prune more than they need |
| Many | Many small twigs broken off | Wind breakage | |
| Many | Many small twigs broken off | Twig pruner, twig girdler (insects) | Rake up and destroy fallen twigs |
| Many | Vertical splitting of bark along the trunk; sudden appearance; no decay evident | Frost cracks or southwest injury | Frost can split thin bark, particularly on young trees on the southwest side of the main stem/trunk; use tree-wrap to protect bark from temperature fluctuations |
| Many | Vertical splitting of bark along the trunk; sudden appearance; no decay evident | Sunscald | Thin-barked trees, e.g., young ones, split when exposed to intense sunlight; use tree-wrap or block sun with board on bright days; avoid heavy fertilization in late summer and in fall |
| Many | Vertical splitting of bark along the trunk; sudden appearance; no decay evident | Lightning injury | Use lightning rod |
| Many | Other trunk damage, not vertical splitting of bark | Mechanical injury, e.g. lawn mower, weed whacker, etc. | Avoid injury to trunk. Mulch in donut shape around the tree, so that mulch does not contact the trunk. Mulch should be in a shallow layer, approximately 2” or less. |
| Many | Large areas of split bark; decay evident in wood | Secondary decay of any of the mechanical damage described above | No chemical controls; if practical provide deep irrigation to trees during drought and fertilize, if recommended by a soil test |
| Many | Large areas of split bark; decay evident in wood | Fungal or bacterial canker (any of several) | Wood decay is often difficult for a remote laboratory to diagnosis; it is advisable to begin with a digital sample; if decay is in the trunk there are no controls |
| Many | Gray or white powdery growth on leaves; leaves may be distorted | Powdery mildew (fungal disease) | Use registered fungicide |
| Many | Black, sooty growth on leaves and/or stems | Sooty mold (fungus that grows on honeydew substance secreted by aphids and other insects) | Sooty molds are not pathogenic to plants, but unsightly and can block sunlight; control insect problem to avoid honeydew |
| Many | Brown dead areas on leaf margins | Leaf scorch | Anything that interferes with adequate water uptake can cause leaf scorch and an abiotic or biotic factor could be the cause. Try to determine/rule out possible abiotic factors, such as establishment stress, lack of adequate water, etc. Submit a sample for a diagnosis. |
| Many | Brown dead areas on leaf margins | Cold injury | Cold damage would be distinguished by a sudden appearance. Look at weather history. |
| Many | Tree or shrub wilted and may have poor color | Wilted and off-color foliage may result from various abiotic (e.g. poor planting, establishment stress, lack of irrigation, etc.) or biotic cause (e.g. root rot, decay in the main stem, girdling roots, vascular disease). | Collect background history of plant to determine if the problem may be abiotic. If no abiotic cause (e.g. overly wet soil conditions, drought, establishment stress, etc.) is identified, then submit a sample for diagnosis. |
| Many | Interveinal yellowing of leaves; no wilting | Nutrient deficiency | Soil test |
| Many | Large, corky galls at base of shrub or on roots | Crown gall (bacterial disease) | Avoid wounding shrubs since that provides an entry point for the crown gall bacterium. Some galls can be pruned out if not on main stem; shrubs may live for many years in spite of a gall. |
| Many | Few or no flowers | Cold injury | |
| Many | Few or no flowers | Improper pruning | Some plants flower only on old wood |
| Many | Few or no flowers | Overfertilization with nitrogen | This stimulates leaf production and reduces flower production |
| Many | Few or no flowers | Shade | Grow plants in proper amount of light for the species |
| Many | Few or no flowers | Incorrect fertility | Soil test |
| Many | Galls on upper branches | Fungal disease (any of several) | Submit sample for laboratory analysis; prune out galled branches |
| Many | Galls on upper branches | Various insects | Most are harmless; prune out galled branches |
| Many | Proliferation of branches at specific points on the plant, forming a “witches’ broom” disease effect | Insect injury | For all of these, the only control is to prune out affected branches |
| Many | Proliferation of branches at specific points on the plant, forming a “witches’ broom” disease effect | Fungal, viral, or mycoplasma | |
| Many | Proliferation of branches at specific points on the plant, forming a “witches’ broom” disease effect | Mistletoe | |
| Many | Pustules containing yellow, orange, or black powdery substance on leaves; may be on both leaf surfaces | Rust (fungal disease) | Fungicides may be useful for management, if warranted, but must be timed appropriately and that depends on the particular rust. Submit sample for diagnosis. |
| Many | Brown, gray, green, or yellow crusty, leaflike growths on trunk and branches | Lichens | Lichens are a combination of algae and fungi; they grow on bark and do not harm the plant |
| Many | Early leaf drop | Can be a result of various environmental stress factors or foliar disease or arthropod problem | Collect background information and try to determine if an abiotic factor is involved. If none is found, then submit for laboratory diagnosis. |
| Many | Browning of tips of conifer needles; faint yellow bands about 1/8” wide at the same location across groups of needles | Ozone injury | No controls; white pine is especially sensitive but individual trees vary in sensitivity |
| Many | General browning of conifer needles | Could be a result of abiotic e.g. (salt injury, gas leak, waterlogged soil, transplant shock, stem girdling roots, soil compaction) or a biotic problem. | Collect background information to try to determine if an abiotic factor could be involved. If an abiotic factor is not identified then any biotic-associated problem would likely be in the main trunk/stems or roots. Pinewood nematode is also a possibility on pines not native to north America. Submit a sample for laboratory diagnosis. |
| Many | General browning of conifer needles | Fungal canker | Check trunk and branches for cankers; prune out affected branches |
| Many | General browning of conifer needles | Mites | Use miticide |
| Many | Sour-smelling sap oozes from cracks in the trunk | Slime flux (bacterial disease) | Avoid wounding the tree, protect tree from other stresses such as soil compaction, and promote tree vigor. |
| Many | Yellow and green mottle or mosaic pattern on leaves; leaves may be distorted | Viral disease | No controls; removal of plant may be necessary if virus is easily spread |
| Many | Sunken cankers on trunk or branches; plant may wilt or have poor growth | Fungal or bacterial canker | Submit sample for laboratory analysis; prune out affected branches well below canker. There are no remedies for cankers on the trunk. |
| Many | Oozing sap on trunk | Normal | Some trees naturally ooze sap |
| Many | Oozing sap on trunk | Environmental stress | Drought or waterlogging can cause trees to ooze excessively |
| Many | Oozing sap on trunk | Mechanical injury | Prevent lawn mower injury, other wounds |
| Many | Oozing sap on trunk | Disease or insect damage | See section on specific diseases |
| Many | Brown leaf spots | Fungal or bacterial disease (any of several) | See section on specific diseases or submit sample for laboratory analysis |
| Many | Chemical injury | ||
| Many | Leaves chewed or completely eaten | Various caterpillars, sawflies, leaf beetles, etc. | Use registered insecticide while insects are small and before damage is extensive; consult PMG |
| Many | Fish scale-like structures tightly attached to leaves, twigs, or branches | Various scale insects | Submit sample for laboratory analysis; use dormant oil |
| Many | Young leaves puckered, curled, or distorted; clear, sticky substance on leaves; clusters of small insects on undersides of leaves | Aphids | Use registered insecticide |
| Many | Leaves off-color with tiny white or yellow spots; may appear dirty due to fine webbing and dust that leaves collect | Spider mites | Use registered miticide |
| Many | Galls (abnormal growths on leaves, stems, or other tissues) | Various insects or mites | There are no chemical controls for gall insects, but the plants will not be seriously harmed |
| Birch | Leaves sparse, especially at top of tree; swollen ridges in bark | Bronze birch borer | Use registered insecticide on bark in mid-May, early, mid, and late June |
| Boxwood | Large portions of shrub turn yellow, bronze or brown; on some leaves, the browning may only occur at leaf margins. | Winter injury | Often the damage is most severe or limited to the top of the boxwood. The symptoms typically appear in the spring. Prune out dead branches but wait until June to be sure branches are dead. |
| Boxwood | Large portions of shrub turn yellow, bronze or brown; on some leaves, the browning may only occur at leaf margins. | Volutella blight (fungal disease) | Prune out affected branches |
| Boxwood | Sectional dieback with leaves turning straw-colored. Blackened woody tissue on lower affected stems just beneath the bark. | Colletotrichum dieback | Submit for laboratory diagnosis. Currently there are no effective disease management tactics for this disease, so removal of diseased boxwood is recommended. |
| Boxwood | Large portions of shrub turn yellow or brown; roots rotted; brown discoloration may be evident in wood when base of trunk is cut open | Phytophthora root rot (fungal disease) | Submit sample for laboratory analysis. Improve soil drainage. Fungicides will not benefit boxwood already showing symptoms, but can protect adjacent boxwood. Use registered fungicide. |
| Boxwood | Large portions of shrub turn yellow or brown; roots rotted; brown discoloration may be evident in wood when base of trunk is cut open | English boxwood decline (fungal disease) | Submit sample for laboratory analysis; only a problem on English boxwood; replace shrub with boxwood other than English |
| Boxwood | Large portions of shrub turn yellow or brown; roots rotted; brown discoloration may be evident in wood when base of trunk is cut open | Root feeding nematodes | Submit soil sample for nematode analysis. |
| Boxwood | Leaf spots leading to severe defoliation; green stems have longitudinal black cankers | Boxwood blight | Submit sample for confirmatory laboratory diagnosis since removal of affected susceptible boxwood (English) is recommended. Plant boxwood blight resistant boxwood to avoid the disease and purchase boxwood from nurseries that participate in the Boxwood Blight Cleanliness Program. |
| Boxwood | Leaves are brown and have tiny black specks on them | Macrophoma leaf spot (fungal disease) (see photo 1K) | Secondary problem; prune out branches damaged by winter injury or disease |
| Boxwood | Leaves blistered; small yellowish maggots inside | Boxwood leafminer | Use registered insecticide in early June |
| Boxwood | Leaves at stem tips cupped | Boxwood psyllid | Use registered insecticide just as new growth starts appearing |
| Boxwood | Tiny white or yellow flecks on leaves | Boxwood mite | Use registered miticide as soon as damage is noticed |
| Cotoneaster | Individual twigs die back, turn black, and have curved tips; sunken cankers may be evident on wood | Fire blight (bacterial disease) (see photo 1L) | Prune out affected branches or remove plant if you do not observe the blackening or curved tips; problem could be drought or root rot; blackening that can be rubbed off is sooty mold |
| Dogwood | Late season brown leaf spots | Septoria Leaf Spot (fungal disease) (see photos 1M & 1N) | Septoria leaf spot is a late season disease that is not a threat to the health of the tree. |
| Dogwood | Tiny purplish spots with tan centers or that appear on leaves and flowers in the spring; leaves may be distorted | Spot anthracnose | This is a springtime disease and can be severe in wet springs, but the problem will dissipate with summer. No controls are needed. |
| Dogwood | Brown leaf and bract spots with purple margins; spots vary widely in size and may blight entire leaves; lower branches die back and whole tree may eventually die; leaves cling to tree in winter | Discula anthracnose (fungal disease) (see photos 1O & 1P) | Use registered fungicide |
| Dogwood | Subtle symptoms of off-color leaves or reddish-purplish to brown areas on leaves. Leaves may become scorched, cupped and drop early. | Powdery mildew (fungal disease) | Often laboratory confirmation is needed since symptoms are subtle. Plant dogwood resistant to powdery mildew. Use registered fungicide |
| Dogwood | Leaves wilt and leaf margins turn brown | Scorch, due to hot, dry weather | Dogwood should be planted in reduced sunlight; it is very susceptible to scorch; irrigate regularly and deeply during establish |
| Dogwood | Leaves wilt and leaf margins turn brown | Fungal canker | No controls; water and fertilize |
| Dogwood | Leaves wilt and leaf margins turn brown | Dogwood borer, dogwood twig borer | Use registered insecticide to protect trees; prune out dead and dying branches |
| Dogwood | Leaves wilt and leaf margins turn brown | Lawn mower injury | |
| Elm | Leaves wilt, curl, turn yellow, and drop off; branches die back | Dutch elm disease (fungal disease transmitted by beetles) | Submit sample of many medium-sized affected twigs for laboratory analysis |
| Elm | Leaves wilt, curl, turn yellow, and drop off; branches die back | Various other fungal wilt disease | Submit sample for laboratory analysis |
| Elm | Leaves wilt, curl, turn yellow, and drop off; branches die back | Phloem necrosis (mycoplasma disease) | Submit sample for laboratory analysis |
| Elm | Leaves eaten between veins and appear lacy; may turn brown and drop off | Elm leaf beetle | Use registered insecticide |
| Hemlock | Resinous bleeding near base of trunk on young (3-7 year old) trees; browning under bark | Bleeding canker | No controls known; cause has not been determined |
| Hemlock | Needles with brown specks, especially near base; fine webbing between needles; foliage gray and dirty | Spruce mite | Use registered insecticide |
| Holly | Dieback of part or all of foliage; blackened roots | Black root rot (fungal disease) | Common problem on Japanese hollies and inkberry holly. Plant hollies that are not susceptible to black root rot. A registered fungicide can be used preventatively, but will not benefit plants already showing symptoms. |
| Holly | Tan winding trails or blotches on leaves; tiny brown dots on undersides of leaves | Leafminers | Use registered insecticide in early June |
| Holly | Leaves with tiny yellow spots; leaves small and off-color | Southern red mite | Use registered miticide |
| Juniper | Tips of branches turn brown; black pimple-like structures can be seen on brown needles or stems | Various fungal tip blights (see photos 2D & 2E) | Submit sample for laboratory analysis; fungicides are registered for some of these tip blights |
| Juniper | Browning of parts or all of plant | Root rot (fungal or oomycete disease) | Submit sample for laboratory analysis; fungicides are registered for Phytophthora root rot |
| Juniper | Browning of parts or all of plant | Winter injury | Prune out dead branches |
| Juniper | Browning of parts or all of plant | Drought | Water deeply during dry periods |
| Juniper | Browning of parts or all of plant | Vole damage | Check for vole tunnels in the soil around plants and signs of vole chewing. |
| Juniper | Brick-red or brown galls form on branches; in spring, orange jelly-like horns form on the galls; galls turn brown with age | Cedar-apple rust (fungal disease) | Controls are not recommended for juniper. Spores from the galls spread to infect apple trees. |
| Juniper | Needles gray, dirty-looking, and covered with tiny yellow specks | Spruce mite | Use registered miticide |
| Lilac | Brown spots appear on leaves in early spring; leaves are distorted, turn black and die; flowers die | Bacterial blight | Prune out affected branches |
| Lilac | Frost injury | Prune out affected branches | |
| Lilac | Stems swollen and cracked near ground; sawdust may be present | Lilac borer | Use registered insecticide on bark in early May and mid-June |
| Lilac | Bark chewed off | European hornet | Carefully destroy the hornet’s nest |
| Magnolia | Twigs with powdery white or tan bumps the size of half a bean | Magnolia scale | Use registered insecticide in early September or dormant oil |
| Magnolia | Marginal browning of older leaves or sometimes all leaves | Winter injury | Water deeply in fall before ground freezes; apply anti-desiccant before winter |
| Maple | Large, irregular grayish blotches on leaves; concentric ring pattern in spots are evident when leaves are held up to light | Zonate leaf spot (fungal disease) | No fungicides registered; rake up and burn or bury fallen leaves; this is a late season disease; fungicides are not warranted |
| Maple | Irregular, shiny black, tar-like spots, about 1/2” in diameter on upper leaf surfaces | Tar spot (fungal disease) | Use registered fungicide; rake up and destroy fallen leaves |
| Maple | Irregular, brown spots on leaves; on Norway maple, brown areas follow leaf veins; tree otherwise healthy | Anthracnose (fungal disease) | Use registered fungicide; rake up and destroy fallen leaves |
| Maple | Irregular, brown spots on leaves; on Norway maple, brown areas follow leaf veins; tree otherwise healthy | Scorch, caused by hot, dry weather | Water tree deeply; Anthracnose can be confused with scorch if the leaf spots have enlarged and coalesced; in early stages, it should be possible to distinguish between the two; scorch is mainly at the leaf margins |
| Maple | Brown, dry areas on margins of leaves only | Scorch, caused by hot, dry weather and/or lack of adequate water | Water tree deeply during drought and during establishment |
| Maple | Leaves on tree suddenly wilt and may turn yellow and drop off; wilt may occur on one side of tree only; tree may die suddenly or decline over a period of years; no external trunk or branch damage evident; some branches may have brown streaks in wood | Verticillium wilt (fungal disease) (see photos 2F & 2G) | Fertilizing heavily with nitrogen sometimes helps the tree to recover: distribute nitrogen in holes rather than on soil surface or grass may die; if tree dies, do not replant in the spot or replant with a species immune to Verticillium wilt; water tree deeply |
| Maple | Leaves on tree suddenly wilt and may turn yellow and drop off; wilt may occur on one side of tree only; tree may die suddenly or decline over a period of years; no external trunk or branch damage evident; some branches may have brown streaks in wood | Drought | Water tree deeply |
| Maple | Tree shows “stagheading”, i.e., top branches die back; leaves discolored and small | Poor site | Maple is a shallow-rooted tree and cannot withstand stresses such as soil compaction, being planted near roads and sidewalks, etc. |
| Maple | Tree shows “stagheading”, i.e., top branches die back; leaves discolored and small | Maple decline, a disease thought to be associated with several pathogens and environmental stresses | Watering and fertilizing may help |
| Maple | Tree shows “stagheading”, i.e., top branches die back; leaves discolored and small | Fungal or bacterial canker | Prune out cankered branches; water fertilize |
| Maple | Cottony egg sacs on twigs and leaves | Cottony maple scale, cottony maple leaf scale | Use registered insecticide when egg sacs first appear |
| Maple | Small red, green, or black globular growths on upper leaf surfaces | Gall mites | No control, but the tree will not be harmed |
| Oak | General yellowing of tree or yellowing sections of tree; no wilting | Iron chlorosis, esp. on pin and willow oak | Soil test; iron deficiency can usually be corrected by adjusting soil pH to 6.0; in severe cases, injections of iron can be made |
| Oak | Puckered, circular areas 1/2” in diameter on leaves; blisters are yellowing at first, then turn brown; leaves may drop | Oak leaf blister (fungal disease) | Rake up and destroy affected leaves |
| Oak | Brown, dead areas on leaves, extending out to leaf margins; no trunk damage evident | Anthracnose (fungal disease) | Use registered fungicide; rake up and destroy fallen leaves |
| Oak | Brown, dead areas on leaves, extending out to leaf margins; no trunk damage evident | Various other fungal leaf spots (anthracnose is probably the most common) | Submit sample for laboratory analysis; rake up and destroy fallen leaves |
| Oak | Brown leaf spots that may progress to general leaf browning and defoliation | Tubakia leaf spot | This is typically a late season disease that can be severe in wet seasons and cause premature defoliation but is not a serious threat to the health of the tree. Rake and remove fallen leaves. |
| Oak | Marginal leaf scorch; eventually branch dieback, progression of dieback varies | Bacterial scorch (bacterial disease) | Often there is a yellow line between brown tissue and green tissue with this disease, but it is not always present. Submit sample for laboratory analysis. No control; progression of the disease varies. |
| Oak | Marginal leaf scorch; eventually branch dieback, progression of dieback varies | Scorch, caused by hot, dry weather or inadequate water | Anthracnose can be confused with scorch if leaf lesions have enlarged and coalesced; if browning is mainly at leaf margins, it is probably scorch or the disease bacterial scorch |
| Oak | Leaves wilt, turn bronze color, and drop off; no trunk damage evident | Dry soil | Water tree deeply |
| Oak | Leaves wilt, turn bronze color, and drop off; no trunk damage evident | Oak wilt (fungal disease) | To date we have not diagnosed oak wilt in Virginia and old reports of the disease have not been confirmed. |
| Oak | Leaves wilt, turn bronze color, and drop off; no trunk damage evident | Soil compaction | Correct compaction; consult certified arborist |
| Oak | Galls on leaves or branches | Gall wasps | Oaks are subject to attack by dozens of species of gall wasps. The tree is not harmed even by heavy infestations |
| Photinia | Circular, gray leaf spots with purplish borders; tiny black specks in centers of spots; defoliation | Entomosporium leaf spot (fungal disease) (see photo 2H) | Use registered fungicide on a regular basis throughout the season; avoid pruning and fertilizing plants in summer |
| Pine | Cream-colored pustules containing bright orange or yellow spores form on needles; sides of pustules are papery in appearance; needles may drop | Needle rust (fungal disease) | Fungicides usually not necessary; remove goldenrods and asters around pines: these are alternate hosts of the fungus |
| Pine | Rough, elongated, swollen areas with yellowish orange color develop on trunk and branches; sap may flow from these cankers; needles turn brown | White pine blister rust (fungal disease) | Submit sample for laboratory analysis; remove all currant and gooseberry bushes in a 1000-foot radius of tree (alternate hosts); prune out cankers |
| Pine | Rough, elongated, swollen areas with yellowish orange color develop on trunk and branches; sap may flow from these cankers; needles turn brown | Various other fungal cankers | Submit sample for laboratory analysis |
| Pine | Black, sooty substance covers needles and stems | Sooty mold, fungus very common on pine, grows on honeydew excreted by aphids and other insects | Not a disease; control aphids with registered insecticide to avoid honeydew on which sooty molds grow |
| Pine | Small, circular spots or bands on needles; some needles may be brown from a spot all the way out to the tip | Brown spot or other needle cast | Submit sample for laboratory analysis; time of fungicide application depends on which disease it is |
| Pine | Needles turn reddish brown and remain attached to tree | Pine wood nematode, esp. on Japanese black pine and some other pines not native to North America | Submit sample of lower branches for nematode analysis; remove and destroy affected trees; control longhorn beetles with registered insecticide |
| Pine | Needles turn reddish brown and remain attached to tree | Dry soil | Water tree deeply |
| Pine | Needles turn reddish brown and remain attached to tree | Other environmental stress; e.g. soil compaction, gas leak | |
| Pine | Needles turn reddish brown and remain attached to tree | Fungal canker | Prune out cankers on branches; there are no controls for cankers that form on the trunk |
| Pine | Needles turn reddish brown and remain attached to tree | Fungal tip blight (any of several) | Submit sample for laboratory analysis |
| Pine | Needles turn reddish brown and remain attached to tree | Mites | Use registered miticide |
| Pine | Needles turn brown from tips of branches back | Fungal tip blight (any of several) | Submit sample for laboratory analysis |
| Pine | Needles turn brown from tips of branches back | Dry soil | Water tree deeply |
| Pine | Tips of needles turn brown; two yellow bands about 1/8-1/4” wide appear across groups of needles; tree is otherwise healthy | Ozone injury, esp. on white pine | Tree will recover if damage is not severe |
| Pine | Clusters of caterpillar-like insects feeding on needles in groups | Sawflies | Use registered insecticide |
| Pine | Leader wilts, droops, and dies | White pine weevil | Prune out infested terminals; spray leader with registered insecticide in early April |
| Rhododendron and Azalea | Fleshy, thick, white galls form on leaves and/or flowers | Azalea leaf and petal gal (fungal disease) (see photo 2I) | Pick off and destroy galls |
| Rhododendron and Azalea | General leaf yellowing on all or part of plant | Improper soil pH | Soil test; rhododendron and azalea require acid soil |
| Rhododendron and Azalea | Nutrient deficiency | Soil test | |
| Rhododendron and Azalea | Small, pale, circular spots appear on undersides of flower petals; spots enlarge and appear white on colored flowers and brown on white flowers; flowers become limp and covered with white, fuzzy spore mass | Ovulinia petal blight (fungal disease), mainly on azalea | Use registered fungicide |
| Rhododendron and Azalea | Marginal leaf browning; leaves curl downward on rhododendron | Winter injury | Very common on rhododendron; always suspect this on samples submitted in early spring |
| Rhododendron and Azalea | Marginal leaf browning; leaves curl downward on rhododendron | Scorch, caused by hot, dry weather | Supply water |
| Rhododendron and Azalea | Marginal leaf browning; leaves curl downward on rhododendron | Salt injury | Do not overapply de-icing salt on sidewalks or drives near shrubs or trees |
| Rhododendron and Azalea | Marginal leaf browning; leaves curl downward on rhododendron | Phytophthora root rot (oomycete disease) (see photos 2J & 2K) | Submit sample (include roots) for laboratory analysis |
| Rhododendron and Azalea | Marginal leaf browning; leaves curl downward on rhododendron | Phytophthora dieback (oomycete disease that attacks stem tips) | Submit sample for laboratory analysis |
| Rhododendron and Azalea | Marginal leaf browning; leaves curl downward on rhododendron | Botryosphaeria dieback (fungal disease) | Very common problem. Submit sample for laboratory analysis; prune out dead branches; consult pruning manual for proper technique when doing routine pruning; the fungus often invades pruning wounds or stressed tissue; protect plants from winter injury |
| Rhododendron and Azalea | Brown spots on leaves | Various fungal leaf spots | Submit sample for laboratory analysis |
| Rhododendron and Azalea | Brown spots on leaves | Physiological leaf spot, cause unknown | Easily confused with fungal leaf spot |
| Rhododendron and Azalea | Leaves with yellow specks on upper surface; black, shiny spots on undersurface | Lace bugs | Use registered insecticide |
| Rose | Black, circular lea spots with feathery edges surrounded by yellow halo; leaves drop | Black spot (fungal disease) (see photo 2L) | Use registered fungicide; plant resistant cultivars |
| Rose | Spots of various colors on leaves that do not fit black leaf spot description | Various fungal leaf spots | Submit sample for laboratory analysis |
| Rose | Various patterns of yellow and green on leaves, including streaks, rings, vein clearing (yellow veins), or blotches | Viral disease | Common on roses; these viruses mainly enter through grafts and are not transmitted from plant to plant; purchase healthy stock; maintain shrub vigor by watering and fertilizing; not necessary to remove shrub |
| Rose | General or interveinal chlorosis | Nutrient deficiency | Soil test |
| Rose | General or interveinal chlorosis | Waterlogged soil | Improve drainage |
| Rose | Branches die back; sunken or swollen discolored areas which may be covered with tiny black specks appear on branches | Various fungal cankers | Very common problem on rose; prune out cankers; no fungicides are registered; prevents stress from other diseases and environmental problems; prune near side shoot or bud; do not leave long pruning stubs |
| Rose | Plants wilt; lower leaves may turn yellow | Dry soil | Supply water |
| Rose | Plants wilt; lower leaves may turn yellow | Verticillium wilt (fungal disease) | Use resistant varieties; do not replant in same area |
| Rose | Plants wilt; lower leaves may turn yellow | Root knot (nematode problem) | Check roots for knots; plant in another area |
| Rose | Plants wilt; lower leaves may turn yellow | Waterlogged soil | Improve drainage |
| Rose | Plants wilt; lower leaves may turn yellow | Transplant shock | Water regularly after transplanting |
| Rose | Flowers wilt, develop spots, or fail to open and become covered with fuzzy, grayish mold | Botrytis blight (fungal disease) | Remove and destroy affected flowers; fungicides for black spot should control this disease |
| Rose | Shoots and foliage have an abnormal red color; stems appear thick and succulent; rapidly elongating shoots; shoots with shortened internodes; stems with an overabundance of pliable thorns; new growth may have many branches that create a witch’s broom (similar to glyphosate injury); distorted or dwarfed leaves (similar to 2,4-D injury); deformed buds and flowers; abnormal flower color; lack of winter hardiness | Rose rosette virus (viral disease) | Virus is spread by eriophyid mites; choose healthy nursery stock without any of the symptoms mentioned here; remove any wild multiflora roses within 100 yards of the landscape; space plants well to allow for mature growth to slow potential of mites from spreading from plant to plant; remove any roses diagnosed with this virus, including the roots to avoid spread. Can be mistaken for glyphosate injury, so laboratory diagnosis is recommended. |
| Rose | Flower buds fail to open; blooms are deformed with brown streaks or spots on petals | Thrips | Use registered insecticide |
| Spruce | Needles on branches near ground turn brown; branches die back; needles may drop or remain attached; dried, white pitch may ooze from bark | Cytospora canker (fungal disease) especially on Colorado and Norway spruce | Prune dead branches back to trunk |
| Spruce | Older, inner needles of branches appear speckled with dull yellowish blotches; later these needles turn brown or purple from tips back and drop; tiny black specks in rows on needles can be seen with a hand lens | Rhizosphaera needle blight (fungal disease) | Use registered fungicide |
| Spruce | Older, inner needles of branches appear speckled with dull yellowish blotches; later these needles turn brown or purple from tips back and drop; tiny black specks in rows on needles can be seen with a hand lens | Stigmina needle cast (fungal disease) | Laboratory diagnosis is needed to distinguish these two fungi, but the control recommendation is the same for both. |
| Willow | Willow is very susceptible to a number of different fungal cankers. See general section for description of canker symptoms. Prune out and destroy cankered branches. There are no remedies for cankers on the trunk. |
Table 6-6: Annual and Perennial Flowers Diagnostic Key
| Crop | Symptoms | Possible Causes | Controls & Comments |
|---|---|---|---|
| Many | Plants wilt; flowers may drop and leaves may turn yellow | Dry soil | Supply water |
| Many | Plants wilt; flowers may drop and leaves may turn yellow | Waterlogged soil | Improve drainage |
| Many | Plants wilt; flowers may drop and leaves may turn yellow | Transplant shock | Do not transplant in heat of day; water regularly after transplanting |
| Many | Plants wilt; flowers may drop and leaves may turn yellow | Verticillium wilt (fungal disease) | Submit sample for laboratory diagnosis; replant new plants in another area |
| Many | Plants wilt; flowers may drop and leaves may turn yellow | Root and stem or corm rot (fungal, bacterial or oomycete disease) | Plant in well-drained soil; destroy affected plants |
| Many | Seedlings wilt, stems turn brown and soft and may be constricted at the soil line | Damping-off (fungal or oomycete disease) | Plant in well-drained soil; avoid planting too early in the growing season; destroy affected plants |
| Many | Plants fail to produce flowers | Wrong season | Plants have specific day length requirements for flowering |
| Many | Plants fail to produce flowers | Cool weather | |
| Many | Plants fail to produce flowers | Insufficient light | Do not plant sun-loving plants in shade |
| Many | Plants fail to produce flowers | Too much nitrogen | Do not over-fertilize; nitrogen stimulates foliage, not flowers |
| Many | Plants fail to produce flowers | Immature plants | Biennials and perennials often do not flower in the first year |
| Many | Plants fail to produce flowers | Undersized bulbs | |
| Many | Plants produce too many small flowers | Plants not disbudded | Some flowers; e.g. chrysanthemum, need to have some buds removed to produce large flowers |
| Many | Tall, “leggy” plant; stem and foliage pale or yellow | Insufficient light | Plant in location where species will receive adequate light |
| Many | General yellowing of leaves; yellowing may be interveinal; plants may be stunted; no wilting | Nutrient deficiency or improper soil pH | Soil test |
| Many | Grayish white powdery growth on leaves | Powdery mildew (fungal disease) | Use registered fungicide |
| Many | Pustules containing orange, yellow, or brown powdery substance | Rust (fungal disease) | Use resistant varieties if available; use registered fungicide |
| Many | Brown, dead spots on leaves | Fungal, bacterial, or foliar nematode disease (any of several) | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Many | Brown, dead areas on margins of leaves | Scorch, due to hot, dry weather | Supply water |
| Many | Brown, dead areas on margins of leaves | Salt injury | Do not plant near walkways or driveways that were treated with deicing salt in winter |
| Many | Flowers wilt or fail to open; grayish mold appears on flowers in moist weather | Gray mold (fungal disease) | Pick off and destroy affected flowers; use registered fungicide |
| Many | Yellow and green mottle or mosaic pattern on leaves | Viral disease (any of several) | Remove affected plants; do not touch healthy plants after diseased ones; control insects |
| Many | Tiny, white flecks on leaves | Ozone injury | |
| Many | Tiny, white flecks on leaves | Spider mites | Use registered miticide |
| Many | Tiny, white flecks on leaves | Thrips | Use registered insecticide |
| Many | Clusters of insects on stems or undersides of leaves; leaves may be curled or distorted | Aphids | Use registered insecticide |
| Many | Leaves chewed or completely eaten | Various insects | Submit insect for laboratory identification; refer to controls for specific insect in PMG |
| Many | Leaves chewed or completely eaten | Slugs | Use slug bait |
| Many | Light-colored tunnels or blotches in leaves | Leafminers | Use registered insecticide |
| Many | Tiny, white-winged insects on undersides of leaves | Whiteflies | Use yellow sticky boards (smeared with grease) to trap insects or use registered insecticide |
| Many | White, cottony masses on leaves or stem | Mealybugs | Use registered insecticide |
| Chrysanthemum | Flowers distorted and abnormally colored; rosetting of florets may occur; yellow and green mosaic, mottle or ring pattern may appear on leaves | Viral disease (any of several) | Remove affected plants; control insects |
| Chrysanthemum | Green-colored flowers instead of normal color; upper branches of flowering stem are yellowish and upright | Aster yellows (phytoplasma disease) | Remove affected plants; weed control; insect control |
| Chrysanthemum | Brown, dead flowers | Thrips | Prune out affected flowers; use registered insecticide |
| Chrysanthemum | Brown, dead flowers | Gray mold (fungal disease) | Pick off and destroy affected flowers; use registered fungicide |
| Daylily | Irregular brown streaks on leaves; yellow streak along midvein of leaf, starting at leaf tip; general leaf yellowing | Daylily leaf streak (fungal disease) | Avoid overhead irrigation; cut back and remove old leaves in fall; keep notes on which varieties are most affected and plant those that consistently look better; use a registered fungicide |
| Daylily | Yellow leaf spots and streaks; powdery, orange pustules associated with spots on lower leaf surface | Daylily rust (fungal disease) | Use resistant varieties; remove old plant debris at the end of the season; use a registered fungicide |
| Geranium | Corky, raised spots on lower leaf surfaces | Oedema, a physiological problem associated with overwatering | Do not overwater; ivy geraniums are especially prone to oedema |
| Geranium | Plants wilt; brown or black rotted area evident at base of stem; brown spots may be present on leaves | Fungal or bacterial root and stem rot (any of several) | Plant in well-drained soil; remove dead plants |
| Geranium | Pie-shaped brown areas or small brown spots with yellow haloes on leaves; rot may be present on lower stems | Bacterial stem rot and leaf spot | Plant in well-drained soil; avoid overhead watering; remove and destroy affected plants and plant debris |
| Gladiolus | White streaked flowers | Viral disease | Destroy affected plants; control insects; do not plant near vegetable garden since two major gladiolus viruses also infect plants in bean and cucumber family |
| Gladiolus | Plants are thin with weak leaves that turn yellowish green; flower spikes are twisted and distorted and may be green | Aster yellows (phytoplasma disease) | Destroy affected plants; weed control; insect control |
| Gladiolus | Plants are thin with weak leaves that turn yellowish green; flower spikes are twisted and distorted and may be green | Poor growing conditions | Aster yellows could be confused with poor growing conditions, but twisted flowers are an indication of disease |
| Gladiolus | Plants stunted; flowers small and faded; leaves yellow from tips back; corm may be rotted; corm discolored internally | Fusarium yellows (fungal disease) | Destroy affected plants; practice 4-year rotation; soak corms in registered fungicide before planting |
| Gladiolus | Plants stunted; flowers small and faded; leaves yellow from tips back; corm may be rotted; corm discolored internally | Various fungal corm rots | Same as above |
| Gladiolus | Plants stunted; flowers small and faded; leaves yellow from tips back; corm may be rotted; corm discolored internally | Waterlogged soil | Improve drainage |
| Gladiolus | Plants stunted and yellowed; corm rotted with blue-green, powdery mold on surface | Penicillium corm rot (fungal disease) | Destroy all affected corms; store corms in cool, dry place before planting; dip corms in registered fungicide before planting; rotate |
| Gladiolus | Sunken, black lesions covered with shiny, varnish-like substance surrounded by raised, brittle rims on corms; after planting, tiny, raised reddish-brown specks appear on leaf bases; leaf specks become soft | Scab (bacterial disease) | Destroy affected corms; rotate |
| Gladiolus | Whitish streaks on leaves; flowers deformed and discolored | Gladiolus thrips | Keep plants well-watered; use registered insecticide |
| Hollyhock | Yellow to orange spots on upper leaf surfaces; small, brown pustules on corresponding lower leaf surfaces | Rust (fungal disease) | Pick off and destroy affected leaves; use registered fungicide; note that symptoms of rust on hollyhock are slightly different from those caused by rust on other plants (described in the general section). This rust does not have an alternate host. Destroy all aboveground diseased plant tissue in the fall. |
| Iris | Leaves turn yellow and wilt; if pulled gently, leaves detach from plant; soft, slimy, smelly rot at base of plant; rhizomes may have holes | Bacterial soft rot, spread by iris borer | Dispose of infested plants in fall; use registered insecticide to control iris borer when plants are 5-6” tall |
| Iris | Oval, watersoaked leaf spots that later turn tan and blight the entire leaf | Cladosporium leaf spot (fungal disease, formerly “Heterosporium leaf spot”) | Remove all old leaf debris in the fall: use a registered fungicide when leaves are 4-6” tall |
| Marigold | Plants wilt; leaves wither; lower stem discolored inside and out | Fungal stem rot | Destroy affected plants; rotate; plant in well-drained soil |
| Marigold | Plants wilt; leaves wither; lower stem discolored inside and out | Fusarium wilt (fungal disease) | Same as for stem rot |
| Marigold | Round, corky galls on lower stem | Crown gall (bacterial disease) | Destroy affected plants; rotate |
| Marigold | Round, corky galls on lower stem | Herbicide injury (growth regulator-type herbicide) | If lawn herbicides are used, apply after wind has died down and do not apply in heat of day; avoid using mulch from herbicide-treated fields, manure from animals fed on herbicide-treated fields or grass clippings from herbicide-treated lawns |
| Marigold | Numerous small brown spots on leaves, stems, and flowers | Alternaria blight (fungal disease) | Use registered fungicide |
| Peony | New shoots wilt and turn black; flowers, buds, leaves, and stems turn brown and leathery; gray fuzzy mold may appear in wet weather | Gray mold (fungal disease) | Prune out affected plant parts; use registered fungicide |
| Peony | New shoots wilt and turn black; flowers, buds, leaves, and stems turn brown and leathery; gray fuzzy mold may appear in wet weather | Phytophthora blight (oomycete disease) | Same as for gray mold |
| Peony | New shoots wilt and turn black; flowers, buds, leaves, and stems turn brown and leathery; gray fuzzy mold may appear in wet weather | Cold injury | |
| Petunia | Wilt; leaves light green or yellowed; stems may have soft rot at base; roots rotted | Root and stem rot (oomycete disease) | Plant in well-drained soil; remove affected plants |
| Snapdragon | Pale, yellow spots appear on upper leaf surfaces; reddish pustules of spores appear on upper leaf surfaces in concentric rings | Rust (fungal disease) | Use registered fungicide; note that symptoms of rust on snapdragon are slightly different from those of rust on other plants (described in the general section); pustules form concentric rings on snapdragon |
| Tulip | Stems are very short and flowers bloom at ground level | Warm spring and/or inadequate winter cooling | Place tulip bulbs in paper bags in fall and chill in refrigerator before replanting |
| Tulip | Light or dark-colored spots on leaves and flowers; spots enlarge to form large, gray blotches; fuzzy brown or gray growth appears on spots during wet weather; leaves and stems are distorted | Botrytis blight (fungal disease) | Destroy affected plants; rotate; do not plant spotted bulbs; use registered fungicide |
| Tulip | Flowers streaked, spotted, or mottled in an irregular pattern; leaves may also be streaked or mottled | Viral disease (any of several) | Destroy affected plants; insect control; do not plant variety Rembrandt near other tulips: its showy streak patterns are caused by a virus that may infect other plants; Parrot tulips also streak but this is genetic and not caused by a virus |
| Zinnia | Small, dark brown, angular spots on leaves and flowers; flowers may be completely blighted | Bacterial spot | Submit sample for laboratory diagnosis; use bleach-treated seed |
| Zinnia | Small, dark brown, angular spots on leaves and flowers; flowers may be completely blighted | Various fungal diseases | Submit sample for laboratory diagnosis; refer to controls for specific disease in PMG |
| Zinnia | Reddish-brown, circular spots with grayish white centers; flowers may also have spots | Alternaria blight (fungal disease) | Use registered fungicide |
- The Ortho Problem Solver Hardcover by Michael D. Smith
Attributions
Prepared by James L. Green, Extension Horticulture Specialist, Oregon State University; adapted for Virginia (2009)
- Diagnostic Keys revised by Elizabeth Bush, Plant Disease Diagnostician (2022), Mary Ann Hansen, Plant Disease Diagnostician (2009, 2022), and Eric Day, Insect Identification Lab Manager, Virginia Tech (2009)
- Sabrina Morelli, Arlington Extension Master Gardener (2021 reviser)
- Elizabeth Brown, Bedford Extension Master Gardener (2021 reviser)
- Adria C. Bordas, Extension Agent, Agriculture and Natural Resources (2015 reviser)
Image Attributions
- Figure 6-1: Normal leaf drop among older leaves nearer to trunk versus abnormal leaf drop from newer leaves towards tip of branch. Johnson, Devon. 2022. CC BY-NC-SA 4.0.
- Figure 6-2: Patterns of dieback on plant canopy. A. Shows canopy death due to urban tree decline B. shows branch death due to dogwood twig borer (Oberea tripunctata). Johnson, Devon. 2022. CC BY-NC-SA 4.0. Includes image 5454691 by Jason Sharman from Bugwood.org CC BY-NC 3.0 US and image 3056077 by James Solomon from Bugwood.org CC BY 3.0 US.
- Figure 6-3: A. Dieback on branch due to cold injury B. Dieback on branch due to Phomopsis blight (Phomopsis juniperivora). Johnson, Devon. 2022. CC BY-NC-SA 4.0. Includes image 5049098 by Joseph OBrien from Bugwood.org CC BY 3.0 US and image 0485003 by David J. Moorhead from Bugwood.org CC BY-NC 3.0 US.
- Figure 6-4: Needle damage. Johnson, Devon. 2022. CC BY-NC-SA 4.0. Includes image 1241649 by Susan K. Hagle from Bugwood.org CC BY-NC 3.0 US and image 1241572 by USDA Forest Service from Bugwood.org CC BY-NC 3.0 US.
- Figure 6-5: Chemical damage from: A. Sulfur dioxide in a consistent pattern across pecan leaf; B. Flouride injury on birch which is concentrated around leaf margins. Johnson, Devon. 2022. CC BY-NC-SA 4.0. Includes image 1505014 by USDA Forest Service – Region 8 – Southern from Bugwood.org CC BY 3.0 US and image 1494137 by University of Georgia Plant Pathology from Bugwood.org CC BY 3.0 US.
- Figure 6-6: Example of fungal leaf sports, photo shows entomosporium leaf spot. Spots usually vary in size, are generally round, and occasionally elongate on stems. (Diplocarpon mespili) on chokeberry leaf. Johnson, Devon. 2022. CC BY-NC-SA 4.0. Includes image 5368844 by Paul Bachi from Bugwood.org CC BY-NC 3.0 US.
- Figure 6-7: Example of bacterial leaf spots on leaf, photo shows bacterial spot of stone fruits (Xanthomonas arboricola pv. pruni) on plum leaf. Johnson, Devon. 2022. CC BY-NC-SA 4.0. Includes image 0162018 by U. Mazzucchi from Bugwood.org CC BY-NC 3.0 US.
- Figure 6-8: Vein clearing, photo shows Tobacco etch virus (Potyvirus TEV) on tobacco leaf. Johnson, Devon. 2022. CC BY-NC-SA 4.0. Includes image 1440033 by R.J. Reynolds Tobacco Company from Bugwood.org CC BY 3.0 US
- Figure 6-9: Mosaic leaf pattern, photo shows alfalfa mosaic (Alfamovirus Alfalfa mosaic virus) on soybean leaf. Johnson, Devon. 2022. CC BY-NC-SA 4.0. Includes image 5605168 by Craig Grau from Bugwood.org CC BY-NC 3.0 US.
- Figure 6-10: The highly invasive spotted lanternfly is a phloem-feeding insect. Image 5524251 from Joseph OBrien, USDA Forest Service, Bugwood.org, CC BY 3.0 US
- Figure 6-11: Examples of cold damage. Johnson, Devon 2022. CC BY-NC-SA 4.0. Includes image 5049093 by Joseph OBrien, USDA Forest Service, Bugwood.org CC BY 3.0 US and image 5363644 by Howard F. Schwartz, Colorado State University, Bugwood.org, CC BY 3.0 US
Living agents such as fungi, nematodes, bacteria, and viruses
Nonliving factors such as nutrient deficiencies and water or temperature stress
Yellowing of normally green tissue
Physical expressions of disease in the host tissue, e.g., changes in color, appearance, integrity, etc.
Structures or products of the pathogen itself on a host plant, for example, mold, fungal fruiting bodies, or bacterial slime/ooze
Growth on the external tissues of a plant
Transport tissue in vascular plants, transports the soluble organic compounds made during photosynthesis to the rest of the plant in a process called translocation
Transport tissue in vascular plants, transports water from roots to stems and leaves (also transports nutrients)

