7 Pulmonary Embolism
Learning objectives
- Describe the factors contributing to the occurrence of pulmonary embolism.
- Describe the pathophysiological consequences and clinical manifestations of pulmonary embolism.
Like any other embolism, an embolus affecting the lung tissue can be made of fat, amniotic fluid, tumor, tissue fragment, or foreign body, but by far the most common cause of pulmonary emboli are blood clots.
The lung contains the first diverging vascular network after the venous system so is particularly vulnerable to deep vein thrombi (DVT) breaking away, traveling through the progressively widening veins, through the right heart, and wedging into the progressively narrowing pulmonary arterial system.
Pathology of Pulmonary Embolism (PE)
About 90 percent of PEs are caused by deep vein thrombi, but at least one of three main predisposing factors (Virchow’s triad) are present in a case of PE:
- Abnormal vessel walls,
- Stagnation of blood, and
- Increased coagulability.
Abnormal vessel walls: Damage to the inner wall of veins causes adherence of blood platelets and activation of clotting factors. Similarly inflammation or trauma of the vein or surrounding area can lead to a risk of local clotting.
Venous stasis: Venous stasis appears to be the most important factor in thrombus formation, and cases of PE are often preceded by periods of immobility (top tip: a USMLE question with a long-haul flight in the stem should raise the PE flag). But other causes of disrupted venous blood flow can elevate the risk.
Hypercoagulability: Any condition that increases coagulability elevates the risk of PE, and most are due to trauma of some form or another or an elevated inflammatory state such as cancer or the postsurgery state. Birth control pills predispose the patient to thromboembolic disease so thereby also increase the risk of PE.
Pathophysiology of PE
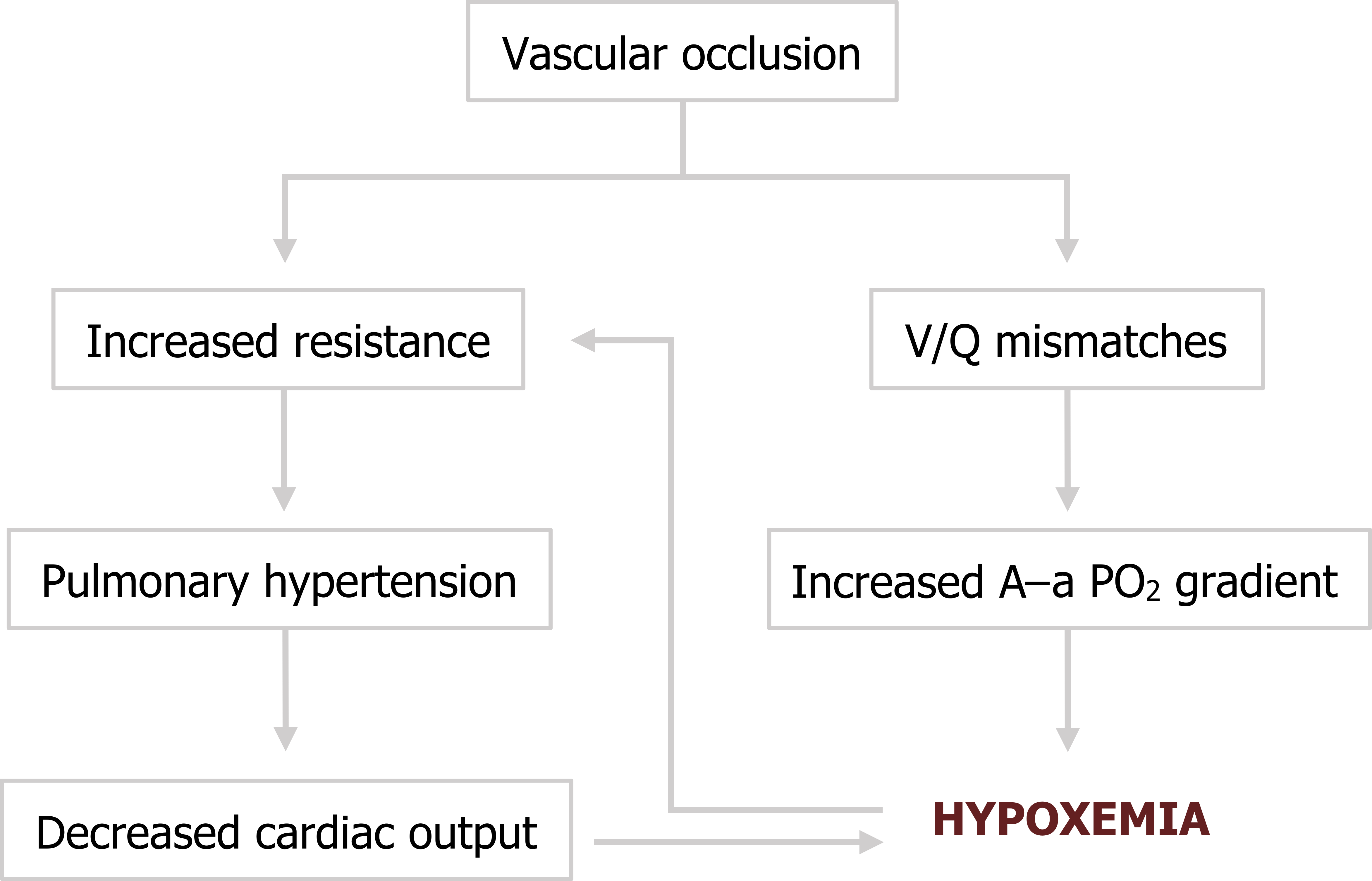
The pathophysiology and clinical severity of PE depend on the number and size of the emboli, so clinical manifestations can be highly variable. In fact, PE is suspected to be much more common than previously thought because of improved detection techniques revealing more small and asymptomatic cases.
Small emboli that can travel further into the vasculature may cause occlusion of relatively small areas of the lung, but with these areas receiving no perfusion and still being ventilated V/Q becomes inappropriately high. Not being able to pass the occlusion, blood will be diverted to other areas of the lung, and consequently cause them to be overperfused, lowering V/Q.
Depending on the size and number of emboli, these V/Q mismatches can produce a widening alveolar–arterial PO2 difference and lead to hypoxemia.
With larger emboli that occlude larger vessels there will not only be a larger impact on gas exchange, but also a more increase in pulmonary vascular resistance. The extreme (and thankfully rare) case is a “saddle” embolus that is large enough to straddle the bifurcation of the pulmonary trunk, obstruct the left and right pulmonary arteries, and lead to immediate hemodynamic collapse. In lesser cases, pulmonary hypertension will overwhelm the thin myocardium of the right ventricle, and as pulmonary arterial pressure approaches right ventricle pressure then cardiac output will fall. This will exaggerate the hypoxemia and cause the pulmonary vasculature to perform its normal vasoconstrictive response to low oxygen tensions that in turn worsens the pulmonary hypertension (summarized in figure 7.1).
Pulmonary infarction, however, is rare, occurring in only 10 percent of PE cases. The lung tissue is supplied by the bronchial circulation so can usually survive the embolism in the pulmonary circulation unless there is preexisting cardiac disease.
Clinical Signs of PE
The clinical manifestations of PE vary widely, from asymptomatic when emboli are small or few, to sudden death when they are large or numerous.
Signs of DVT may precede PE cases, such as leg pain, venous swelling, or warm skin over the thrombus site, but these are present in less than half of patients. Once instigated, the presenting symptom of PE is usually dyspnea but can also include chest pain. There can be clinical clues in the dyspnea as it has a rapid onset and is disproportionate to any initial clinical findings. The nature of its onset also tends to generate significant anxiety. Chest pain can start as anginal but then become more pleuritic.
Hemoptysis is less common but an important symptom. In severe cases involving massive PE tachypnea, tachycardia and cyanosis are usually present.
Because of the mechanical and metabolic strain on the heart, cardiac manifestations, including arrhythmia, acute cor pulmonale, or signs of cardiac failure or shock, may be detected with an increased difference between alveolar and arterial PO2s.
The chest x-ray will appear normal, unless there are complications such as pleural effusion, atelectasis, or pulmonary infarction. CT angiography is used to detect the presence of a PE when suspicion is high. When suspicion is lower, labs will include a D-dimer test (a protein fragment produced by a dissolving clot), which, if negative, can help rule out the presence of a PE, but if positive would require further investigation (e.g., CT angiography) for confirmation.
References, Resources, and Further Reading
Text
Farzan, Sattar, with Doris L. Hunsinger and Mary L. Phillips. “Chapter 21.” In A Concise Handbook of Respiratory Diseases. Reston, VA: Reston Publishing Company, 1978.
Husain, Aliya N. “Chapter 15: The Lung.” In Robbins and Cotran Pathologic Basis of Disease, 9th ed., edited by Vinay Kumar, Abul K. Abbas, and John C. Aster. Philadelphia: Saunders, an imprint of Elsevier Inc., 2015.
West, John B. “Chapter 6: Vascular Diseases.” In Pulmonary Pathophysiology: The Essentials, 7th ed. Baltimore: Lippincott Williams & Wilkins, a Wolters Kluwer business, 2008.
Figures
Figure 7.1: Pathophysiology of pulmonary embolism. Grey, Kindred. 2022. CC BY 4.0. https://archive.org/details/7.1_20220203

