8 Immunological Diseases of the Lung
Learning objectives
- Compare and contrast the mechanisms and manifestations of hypersensitivity pneumonitis, Goodpasture’s syndrome, systemic lupus erythematosus, rheumatoid disease, progressive systemic sclerosis, and polymyositis.
The lung is no different from any other organ in that it is susceptible to disorders of the immune system. One might argue that it is surprising that the lung does not encounter more problems, given the lung’s exposure to the environment and the myriad antigens it encounters. Before we start looking at a few specific disorders, let us quickly review the four mechanisms through which the immune system might disrupt lung tissue.
A type 1 reaction, or immediate hypersensitivity (table 8.1), is a result of overexpression of IgE (table 8.1). When an antigen binds to the overexpressed IgE on the surface of mast cells, the cell releases histamine and leukotrienes that in turn induce an inappropriate or exaggerated inflammatory response. Allergic asthma is an example of a type 1 reaction.
A type 2 reaction, or antibody-dependent cytotoxic reaction (table 8.1), is the result of a circulating antibody reacting with a component of a cell or tissue. The formation of this inappropriate immune complex results in the cell or tissue being flagged for attack by the immune system. Goodpasture’s syndrome is an example of a type 2 reaction.
A type 3 reaction, or immune complex reaction (table 8.1), is the result of an immune complex forming either locally or circulating from elsewhere and then depositing itself in tissue; the immune complex then instigates an immune system attack that involves the tissue. Pulmonary vasculitis can be caused by type 3 immune disorders.
Lastly, a type 4 reaction, or cell-mediated hypersensitivity (table 8.1), is caused by a population of hypersensitive T cells whose response to an antigen is exaggerated and leads to the proliferation of that cell population and the release of lymphokines to induce an inflammatory response. While this pattern is the same as the normal response to many infections, the magnitude of the response is inappropriate and can lead to pathological changes, such as allergic alveolitis.
| Type of immune mechanism | Visual | Description |
|---|---|---|
| Type 1 |  |
- Antigen–IgE interaction - Mast cell release of histamine, leukotrienes - E.g., allergic asthma |
| Type 2 |  |
- Component of cell acts as antigen - Antibodies bind and cell attacked - E.g., Goodpasture's syndrome |
| Type 3 |  |
- A remotely or locally formed immune complex embeds into tissue - E.g., pulmonary vasculitis |
| Type 4 |  |
- A sensitized T-lymphocyte responds to arrival of an antigen with proliferation and release of lymphokines - E.g., allergic alveolitis |
Table 8.1: Types of immune mechanisms involved in lung tissue injury.
With those mechanisms defined, let us look at some specific disorders.
Hypersensitivity Pneumonitis
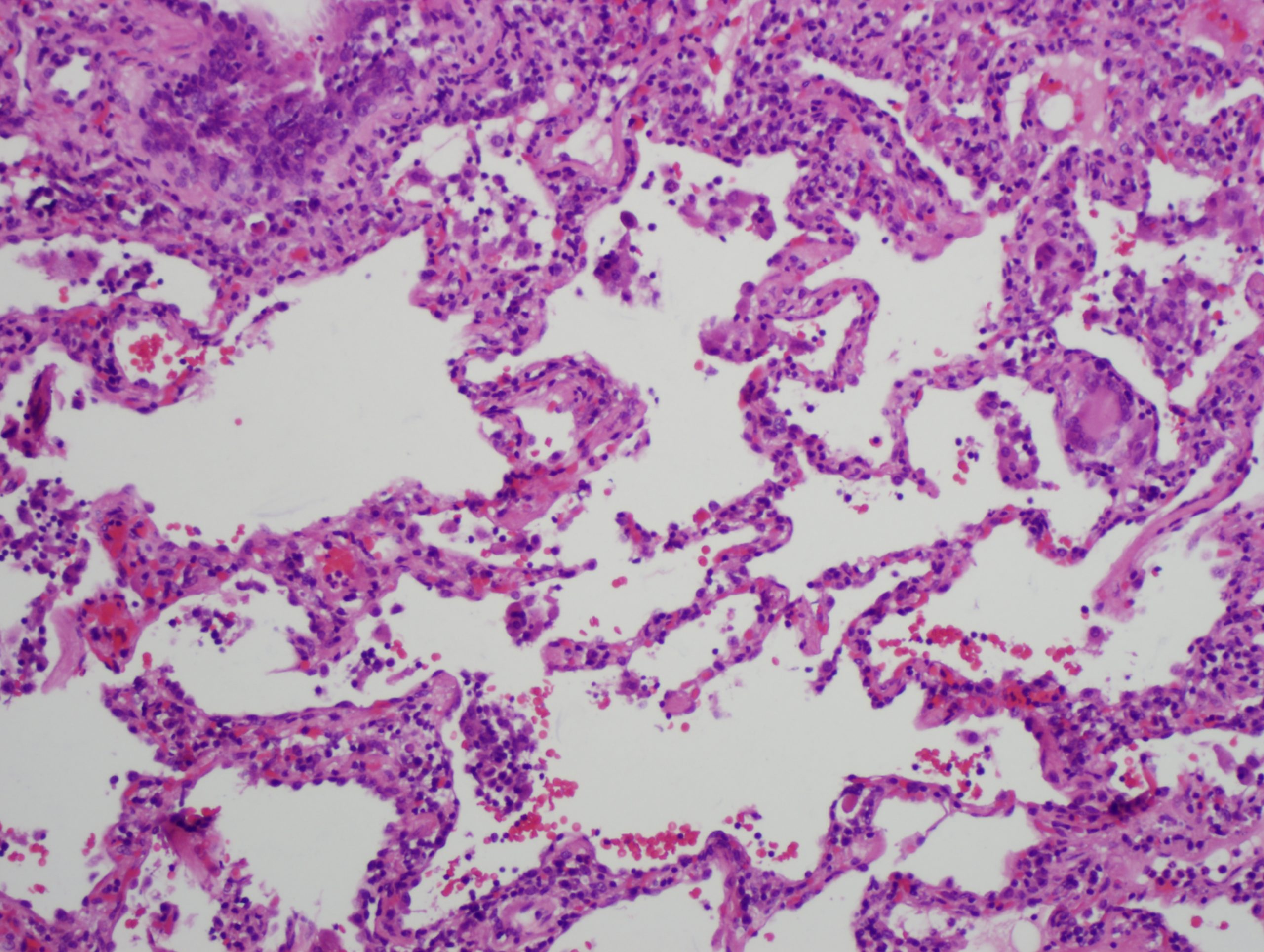
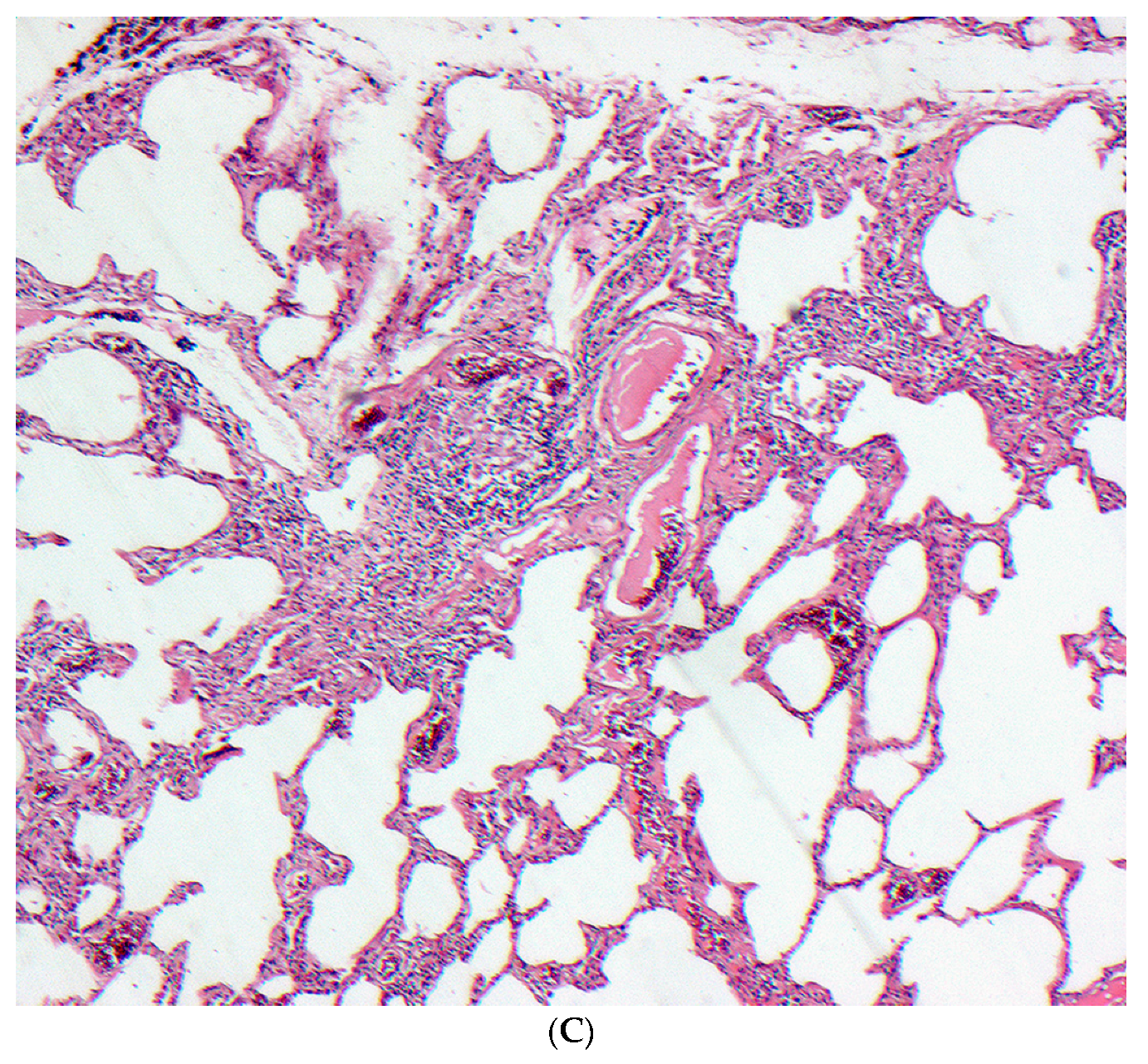
In the acute phase, lymphocytes and macrophages infiltrate the alveolar walls and loose granulomas can form. The presence of multi-nucleated giant cells (figure 8.2), are helpful in diagnosis, but more typical is a dramatic rise in lymphocyte count in BAL fluid, particularly CD8+ cells.
In the subacute phase there is evidence of interstitial thickening and the onset of fibrosis can be seen. Involvement of the bronchioles is seen with evidence of chronic bronchiolitis. The chronic form is marked by significant fibrosis (figure 8.3), to the extent it is indistinguishable from pulmonary fibrosis with distinctive fibrotic patterns and all the hallmarks of restrictive lung disease.
Clinical signs
How hypersensitivity pneumonitis presents is somewhat dependent on the form of exposure the patient had.
With brief but heavy exposure, an acute presentation of pneumonitis will present with fever, malaise, cough, and dyspnea. Physical exam confirms the fever and tachypnea and cyanosis can reflect the severity of the response. Bibasilar rales are often present, but unless a type 1 hypersensitivity arises then wheeze is usually absent (but you might note here that concurrent allergenic asthma is not beyond the realms of possibility). This acute form usually resolves within a couple of days, but can reoccur when the patient is exposed to the causal agent again.
When exposure is light but prolonged, the onset of hypersensitivity pneumonitis is more insidious and clinically challenging. The patient will describe a slowly progressive cough and developing dyspnea, also weakness and weight loss. This form of onset is common with continuous exposure to organic dust. With this longer time line the patient is not usually aware of the symptom’s relation to occupation and exposure persists until diffuse pulmonary fibrosis is established. At this point the signs and symptoms are related to respiratory insufficiency.

