5 Pain, Sentience, and Animal Welfare
Learning Objectives
- Describe the nervous system components involved in the perception of pain in fish.
- Apply criteria for pain to assess whether an animal perceives pain.
- Describe different criteria used to judge sentience.
- Create and critique ethical arguments for the treatment of fish.
- Judge conditions that are most likely to cause fish pain and suffering and actions to alleviate pain and suffering.
- Distinguish between three alternative views on animal welfare.
- Describe specific actions that can be taken to improve welfare of fish.
5.1 Relevant Questions
Fish are not stupid creatures. In fact, fish are socially complex, with highly developed learning abilities (Brown 2015). Fish feel pain and suffer as a consequence, and we must carefully examine welfare, use, and fishing practices. Scientists have questioned the outdated perspective that fish cannot have consciousness as their brain morphology is too simple and lacks the cerebral cortex present in humans. Yet, denial of fish pain perception prevails despite many recent, fascinating discoveries that demonstrate that fish do experience and remember exposures to noxious stimuli in a fashion that is far more complex than mere reflex. Consequently, there are many lively discussions on how we should treat fish.
Think of all the ways that you use fish in your life. Perhaps you enjoy sportfishing or keep tropical fish in aquariums. Maybe you harvest live fish for bait fishing. You may prefer to purchase fresh fish from the local seafood market. You may enjoy watching fish in public aquariums or by SCUBA diving. Or perhaps you identify with Santiago, the aging fisherman in The Old Man and the Sea, who struggles to reel in a giant marlin. Humans use fish for sport, food, pets, business, education, scientific research, and many other purposes (Olden et al. 2020). Whenever we use fish for any reason, we need to ask certain questions: How might our actions influence fish? Do fish feel pain? Do fish suffer? Are fish aware of their actions? Do fish in captivity have what they want? Is the fish healthy? How can we balance fish welfare with the benefits humans get from fish?
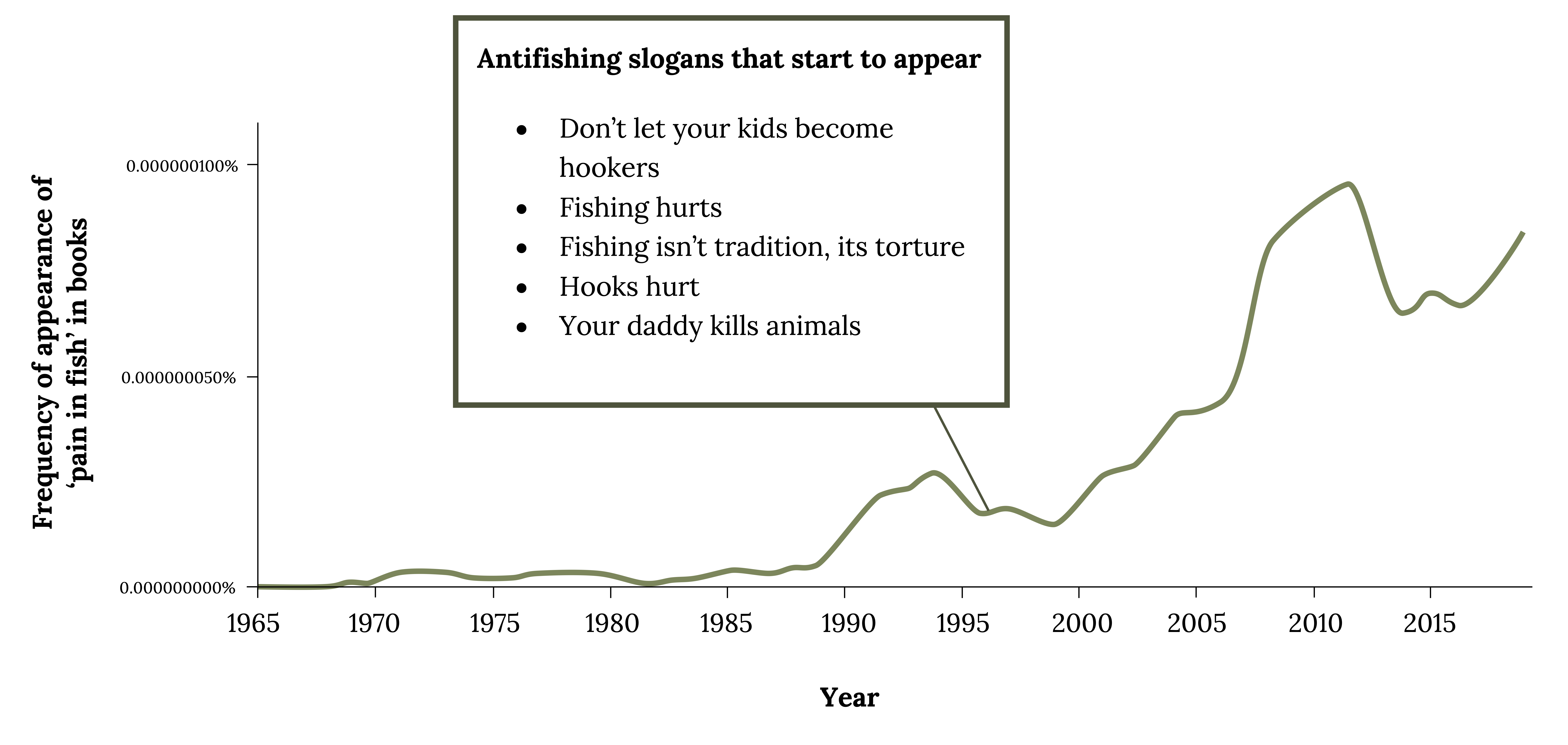
Although anglers and others have long pondered these questions, scientists began systematic investigations of these questions only within the last 50 years (Vettese et al. 2020). According to the International Association for the Study of Pain, pain is “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” (Raja et al. 2020). What causes “unpleasant” and “emotional” responses in fish is a difficult scientific question to answer, long neglected by researchers. Early laws that regulated how animals are used in experiments excluded cold-blooded animals. The Health Research Extension Act of 1985 (PL 99-158, 1985) and the Animals (Scientific Procedures) Act (1986) gave protections to fish and further stimulated the science of animal suffering to include fish (Dawkins 2008; Braithwaite 2010). After the first study investigating whether fish feel pain was published (Sneddon 2002; Sneddon et al. 2003a), many strong feelings and debates emerged (Figure 5.1). This chapter presents the factual evidence and philosophical views and practices related to minimizing pain and suffering in fish.
Our personal decision making about how to treat fish involves reflecting on facts, intuitions, and moral principles about pain and suffering in fish. As such, we judge the relevance of both factual or descriptive statements as well as relevant moral principles. In practice, these reflections are difficult and demand that we participate in dialogue and debate with others who may disagree with our views. Disagreements may be over acceptability of moral principles or over the facts about consequences of different welfare measures on fish consciousness and suffering. Ethical considerations of fish involve application of existing normative theories (Meijboom and Bovenkerk 2013; Michel 2019; Veit and Huebner 2020), resulting in alternative perspectives (List 1997; Allen 2013; Rose et al. 2014; Key 2015, 2016a, b). If this was easy, someone would have done it already.
Who hears the fishes when they cry?
―Henry David Thoreau, A Week on the Concord and Merrimack Rivers, 1849
5.2 Pleasure and Pain Perception
Jeremy Bentham was one of the great thinkers in moral philosophy. He developed the theory of utilitarianism as the basis for law in 18th century England. In Bentham’s view, laws should serve to maximize the interests and preferences of all individuals. The foundation of utilitarianism held that pleasure is the only good, and pain, without exception, is the only evil. In response to creating a penal code regarding cruelty to animals, Bentham wrote, “The question is not, Can they reason? nor, Can they talk? but, Can they suffer?” This proposition formed the beginnings of utilitarian arguments for the ethical treatment of animals (Singer 1975).
Until recently, few scientists asked the question, “Do fish feel pain?” Here I highlight some key findings from studies on fish pain that asked three questions: (1) Do fish have the necessary receptors and nerve fibers to detect painful events? (2) Did a potentially painful stimulus trigger activity in the nervous system? (3) How did the experience of a potentially painful event affect the behavior of fish and decisions made? (Sneddon et al. 2003a; Braithwaite 2010).
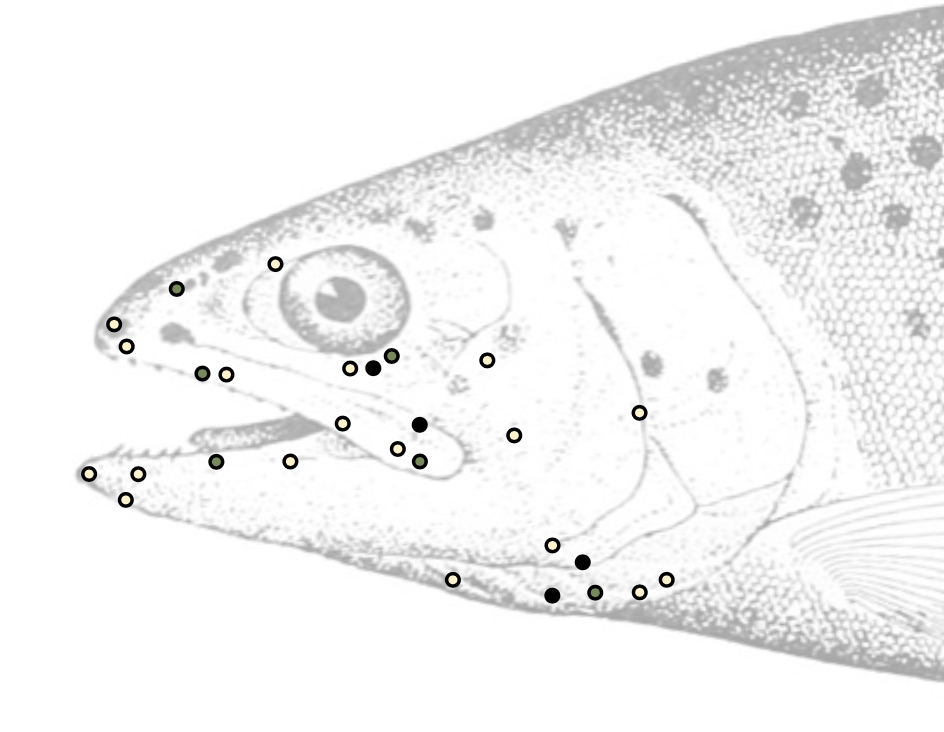
Do fish have receptors to detect painful events? Nociceptors, the sensory receptors to detect noxious stimuli, are present in mammals, birds, reptiles, amphibians, lampreys, and bony fish. Even far distant animal groups, such as leaches, sea slugs, and fruit flies, have nociceptors (Whitear 1971; Matthews and Wickkelgren 1978; Sneddon 2002; Smith and Lewin 2009). Strangely, a few studies suggest that sharks and rays seem ill equipped to detect noxious stimuli, although more studies are needed (Snow 2003). The first descriptions of pain receptors in bony fish revealed that they were similar in size and structure to those observed in birds and mammals (Schnitzler and Ploner 2000; Sneddon 2002; Sneddon et al. 2003a, 2003b, 2018; Sneddon 2019). Nociceptors mapped on the head of Rainbow Trout indicate where pressure and chemical stimuli are detected (Figure 5.2).
Does the painful stimulus trigger activity of the nervous system? Scientists measure the electrical signals in nerves to determine if they respond to stimuli. They also use a technique called electroencephalography (EEG) to record electrical activity of the brain. For example, EEG was used to determine loss of and return of consciousness following stunning in studies designed to discover the quickest methods for killing fish (Robb et al. 2000). When the pain receptors in trout were stimulated by mechanical means, heat, or acid, activity in nerve fibers was recorded (Ashley et al. 2007). The painful stimulus triggered a quick reflex reaction. The second response to the painful stimulus requires processing in the brain and leads to the third question.
How did the experience of a potentially painful event affect the behavior of fish and decisions made? Think about pain that you have experienced. Minor pain may be tolerated without much affect. However, chronic or intense pain will be a priority concern and cause you to change your behavior. Therefore, the third question asks whether behavior or decision making changes after a potentially painful event. Trout responded to acid or bee venom applied to the lips by rubbing their lips against the gravel at the bottom of the holding tank (Sneddon et al. 2003a, 2003b). In other experiments in which Rainbow Trout were exposed to noxious stimuli, they stopped feeding and showed lower antipredator behaviors and lowered aggression with other Rainbow Trout (Ashley et al. 2009). The adverse effects were relieved by painkillers, such as aspirin, morphine, and lidocaine (Lopez-Luna et al. 2017; Sneddon 2015, 2019; Sneddon et al. 2018a).
Questions to ponder:
What is the principal evidence for concluding that fish can experience pain? Explain the questions and methods for the scientific studies. Would you expect all types of fish to have the same types, locations, and number of pain receptors? Why?
5.3 Are Fish Sentient?
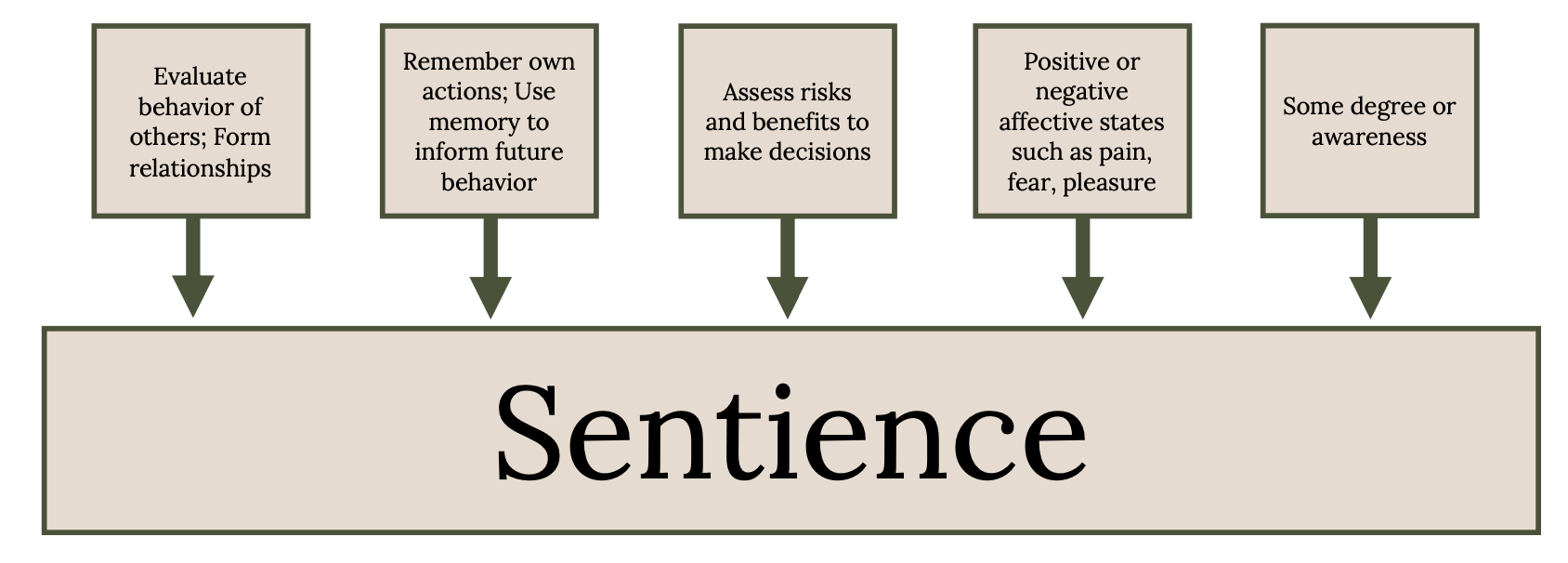
In judging whether an animal deserves respect or protection, what matters morally is whether an animal is sentient and can be benefited or harmed by our actions (Singer 1975, 2010, 2011; Horta 2018). A sentient being can detect and sense external stimuli and is aware of how this perception alters its mental status. The concept of sentience provides the foundation for the animal welfare and animal rights movements (Regan 1983). The moral reasoning follows the argument: (1) If a being is sentient, then it deserves serious moral consideration; (2) fish are likely to be sentient; (3) therefore, fish deserve serious moral consideration (Lund et al. 2017). Whether an animal is sentient is based on the following five capabilities (Figure 5.3; Broom 2014).
Evaluate the actions of others in relation to itself and third parties (i.e., form relationships within and between species).
Anyone who has ever kept fish in aquariums knows that fish will quickly remember who feeds them and gradually habituate to the presence of the person doing the feeding. Fish develop relationships with their aquarium feeder. Fish develop relationships with other fish. We see behavioral displays and dominance in a small group of fish, especially when fish are in captivity. Cooperative relationships are observed in breeding cichlid fish, which care for their young offspring. Even different species, such as moray eels and grouper, may form cooperative hunting behaviors to enhance feeding success (Bshary et al. 2006). They can evaluate hierarchies from a third-part perspective through transitive inference.
Remember some of its own actions (the cognitive ability to learn and recall those memories that should influence future behavior).
In captivity, fish will quickly learn where the food is coming from; if the location changes, fish will learn a new location. In fish farms, fish learn to operate demand feeders. Fish also learn by watching each other (social learning) and avoid fighting with larger, stronger individuals. Many species of fish will return to their home after being experimentally displaced. They learn spatial arrangements in the environment and can remember the whereabouts of different locations and learn migration routes from watching other more experienced fish. Fish learn to avoid nets and hooks and retain that memory for almost a year. They also learn the location of dangerous places and avoid them. More studies on fish learning are highlighted in section 5.6.
Assess risks and benefits (make decisions based on the information available externally and its own subjective state).
Fish in the wild are always at risk of being eaten by a larger predator. If all behaviors were instinctive, the amount of risk-taking behavior would be constant, but that is not the case. In a controlled experiment, juvenile sea bass with higher metabolic demands were more likely to take risks after being deprived of food (Killen et al. 2011). Their behavior changed because the motivation (and benefits) of feeding when very hungry outweighed the potential risk of predation (i.e., they prioritized food over predation risk). Therefore, the risk taking depended on the relative benefits and was not a simple stimulus-response reflex. Fish behavior is often guided by the risk sensitivity: they are constantly attempting to balance the risk of certain behaviors (such as exposure to predators while feeding) with the expected benefits (increased feeding leads to growth and reproduction).
Have some feelings (positive or negative affective states such as pain, fear, and pleasure).
We understand and regularly speak of human emotions, such as fear, anxiety, grief, love, happiness, and pain. We can see these emotions in the faces of other humans. The idea that fish have feelings is often met with a response of disbelief. Whether fish have feelings or emotions was not studied because most behaviorists believed responses to stimuli, such as presence of a predator, was instinctive and not related to the emotion of fear. Discerning whether a fish has feelings is challenging, in part because fish live in environments that make it difficult to observe. Yet, fish need to experience pain, fear, and other feelings in order to respond effectively to their environment and survive (Darwin 1872; Millot et al. 2014; Cerqueira et al. 2017).
Fear is a feeling that protects animals from danger. The flight or fright physiological response is a conservative trait in vertebrates. Brains of fish and mammals have homologous structures that process fear stimuli and cause consistent responses. Fish such as Siamese Fighting Fish and zebra fish respond to antidepressant drugs by reducing aggression (Dzieweczynski and Hebert 2012; Theodoridi et al. 2017). These studies demonstrate that fish exhibit responses similar to those observed in humans and that these responses are controlled by the same neurotransmitters.
In addition to fear, fish are capable of positive and negative moods. Recently, ethologists tested whether Convict Cichlid fish, a monogamous fish, showed a negative mood (pessimistic) when partnered with a nonpreferred mate (Laubu et al. 2019). These findings demonstrated that fish experience similar emotions to humans. Serotonin plays a role in emotions in all vertebrates; zebra fish are extensively used to test new medications for anxiety and depression (Pittman and Lott 2014). Play behavior was long deemed to be a trait only exhibited in mammals. To study play in fishes, play was defined as “repeated, seemingly non-functional behavior differing from more adaptive versions structurally, contextually, or developmentally, and initiated when the animal is in a relaxed, unstimulating, or low stress setting.” Behaviors of fish that fit the definition of play include leapfrogging, balancing twigs, batting around balls, jumping into the air, and striking a self-righting thermometer (Burghardt et al. 2015).
Have some degree of awareness (often termed consciousness).
The ability to recognize oneself in a mirror is a rare capacity, once believed to be restricted to great apes, elephants, dolphins, and magpies (Gallup 1970; Plotnick et al. 2006; Prior et al. 2008; Reiss 2012). If an animal recognizes that the image in the mirror is its own, it will cease to respond to the reflection socially and will recognize changes over time. The mirror test is a long-standing test of self-awareness (Gallup 1970), and until recently, few studies tested self-recognition in fish. When manta rays were exposed to the mirror test, they spent more time when a mirror was present in their holding tank, especially in the first ten minutes of the experiment. While visually oriented to the mirror, manta rays made unusual or repetitive behavior, including bubble blowing and atypical social behaviors (Ari and de Agostino 2016). When exposed to a mirror, the Cleaner Wrasse (Labroides dimidiatus) first interacted aggressively as if seeing a rival, but aggressive reactions decreased over time. Instead, it showed atypical behaviors. After individuals were given a visible mark, they would posture in front of the mirror in order to view the location of the mark. Compared to controls with marks that were not visible, marked Cleaner Wrasse spent significantly longer in postures that would allow them to observe color-marked sites in the mirror reflection (Kohda et al. 2019). These findings that fish “passed” the mirror test were surprising to most scientists. It is still unclear whether scientists will accept the findings or question the mirror test and seek alternative tests for cognitive abilities of fish (de Waal 2019; Vonk 2020).
It is difficult to characterize what nonhuman animals are thinking about in relation to others, feelings, or awareness because they do not use a language that humans understand. Therefore, the evidence of sentient abilities in fish often comes from studies of fish behavior (Brown 2015; Sneddon and Brown 2020). In the study of fish behavior, scientists attempt to understand the thoughts of fish from manipulative studies that provide fish with choices and rewards. It’s a neat way of allowing fish behavior to tell scientists what the fish is thinking. From many recent studies of fish cognition, patterns are emerging to support the five criteria for sentience in fish (Sneddon and Brown 2020).
Questions to ponder:
Think about a fish species for which you have some familiarity. Does this fish exhibit some or all of the five capabilities that are criteria for sentience? If you are uncertain, how might you test the fish for one or more of these capabilities? Link to https://scholar.google.com and search for “fish_name” AND “pain” to see if any scientific studies have been published.
5.4 Skeptics and the Pursuit of Empathy
Since the first studies of fish pain, many skeptics have questioned the finding that fish feel pain and suffer and have opposed the need for regulations governing the welfare of fish (Rose 2002; Rose et al. 2014; Key 2015; Diggles and Browman 2017; Browman et al. 2019). Unlike certain mammals, fish lack a familiar face and voice that reveals emotional cues, and they lack nonhuman charisma that motivates advocates (Lorimer 2007). In response to the arguments of skeptics, Sneddon et al. (2018b) note that (1) “Skeptics still deny anything beyond reflex responses in fishes and state that they are incapable of complex cognitive abilities”; (2) “Processing is not restricted to hindbrain and spinal reflexes as skeptics have suggested”; and (3) “Widespread calls for use of the precautionary principle have been called into question by skeptics”—for example, “We should abandon the precautionary principle because the costs to industry would be too high.”
The “no cortex, no cry” argument, the dominant argument of the skeptics (Smith 1968; Rose 2002; Key 2016a; Dinets 2016), maintains that (1) If x feels pain, then x has a neocortex; (2) Fish do not have a neocortex; (3) Therefore, fish do not feel pain.
The counterargument postulates that fish depend on different neural pathways for pain processing that closely parallel those of the amygdala and hippocampus in mammals (Agetsuma et al. 2010; Michel 2019). Basic features of the forebrain (i.e., basal ganglia) involved in decision making, behavior, and rewards are similar in mammals and lampreys, a vertebrate lineage that diverged 560 million years ago (Grillner and Robertson 2016). While the brain of fish is smaller and less structured than the brain of mammals, there is high variation in brain structure among different fish species. Brain functions and neural circuits in fish, though not homologous to the mammalian brain, are complex enough to support phenomenal reasoning and consciousness (Brown et al. 2011; Woodruff 2017).
Charles Darwin first explored the notion of evolutionary continuity and emotions and believed that if humans feel emotions and can suffer, then so too can other animals, but their feelings are not necessarily identical (Darwin 1872). Although scientists have accumulated much evidence that fish fulfill Brown’s criteria for sentience, denial of sentience in fish persists. At the risk of oversimplifying the many writings by those denying sentience in fish, I offer two views often presented. First, many criticize the experiments and argue that scientists have yet to falsify the null hypothesis that “fish do not feel pain” or claim that pain is fundamentally different in nonhuman animals (Key 2016b; Browman et al. 2019). The other common argument is often a “slippery slope” fallacy that asserts that relatively small steps in protecting animals will culminate in significant restriction or bans in certain fishing sectors.
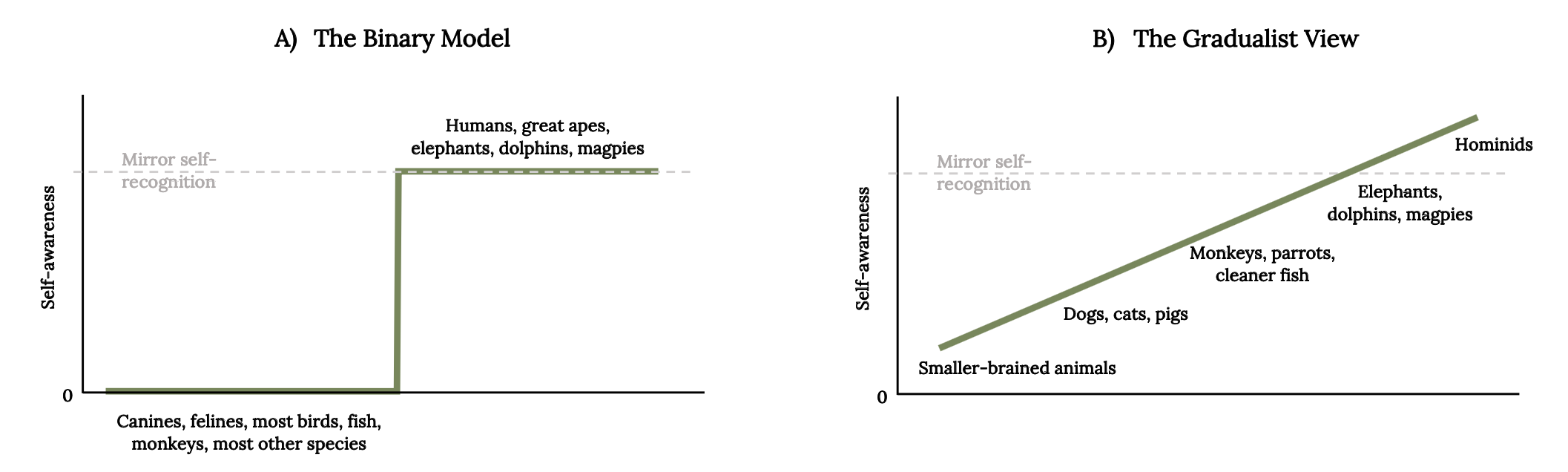
With the emergence of studies on fish consciousness, scientists have questioned whether there is a distinctive line between sentient and nonsentient animals (de Waal 2019; Vonk 2020). Studies of behavior and cognition in fish point to the need for more valid tests for cognitive abilities of fish. Sentience is typically treated as a property that organisms either have or do not have. Alternatively, organisms may possess varying degrees of sentience (Figure 5.4) that influence moral considerability (Veit and Huebner 2020). The controversy over sentience opens a new challenge of understanding the basis for empathy across different species. As a human society, we are struggling to understand what knowledge may lead to actions of care for others (Adriaense et al. 2020).
Questions to ponder:
Regarding pain and sentience in fish, do you feel empathy for fish? Does your need for seafood to eat eclipse sentience? How do you reconcile findings about fish sentience and your sense of moral obligation to making a difference in lives of fish?
5.5 Learning in Fish
Numerous studies support the hypothesis that fish are intelligent, highly social animals. As expected, fish show variation in learning abilities. Fish are capable of learning because they have high-order capabilities, including awareness, reasoning, and consciousness. Yet, popular media are not kind to fish. Dory, the regal Blue Tang in the movie Finding Nemo, is a caricature of the forgetful fish with a short-term memory. In contrast, recent studies tell us that certain fish have long-term memories comparable to other vertebrates (Brown 2001; Brown and Laland 2003, 2011). Fish can recognize one another, learn from dominance relations, use tools, cooperate with other fish, develop cultural traditions, and even have distinctive personality traits. Examples from a few significant experiments reveal impressive memory and abilities to learn.
Behaviors observed in fish reveal their memory and learning. Transitive inference is the ability to infer a relationship between items that have not been previously directly compared. In humans, children around the age of five can infer that if John is taller than Mary, and Mary is taller than Sue, then John is taller than Sue. In one experiment, a male cichlid fish, which is aggressive with other males, was able to observe fights between pairs of male cichlids. Let’s assume the individual cichlid watches as combatant A beats combatant B, B beats C, and C beats D. If the cichlid is now placed in a chamber with A and D, would it avoid either cichlid? If the cichlid avoided A more than D, it has deduced the dominance relationship, even though it never observed the two fish together. This is an example of transitive inference, which requires conscious awareness of the relationships (Grosenick et al. 2007).
In another experiment, rainbow fish learned to escape from a net trawled through an experimental tank and remembered the information for 11 months (Brown and Warburton 1999; Brown 2001). This length of memory was similar to that observed by Common Carp. After capture by hook and line, Common Carp learned to avoid baits presented on hooks and remembered this experience for many months. When foraging in food patches where previous hooking events took place, carp change behavior and spit out baited hooks without being hooked (Klefoth et al. 2013). Common Carp do not have to be captured in order to learn this lesson. Individuals that observed the hooking, struggle, and release of other carp, avoided baits on hooks seven days after the experience (Wallerius et al. 2020).
Tool use was long considered a defining feature separating humans from all other species. Our human perception of “tools” creates difficulty for fish, which have no grasping appendages. Furthermore, the watery environment is more viscous and buoyant, which restricts the mechanical forces involved in operating a “tool.” Studies on cognition in nonhumans necessitated a new definition of tool use that required that the animal “must directly handle an agent to achieve a goal.” Suddenly, many behaviors indicated that some fish were tool users (Keenleyside and Prince 1976; Keenleyside 1979; Coyer 1995; Bshary et al. 2002; Paśko 2010; Jones 2011; Bernardi 2012; Brown 2012). Brown Hoplo Catfish (Hoplosternum thoracatum) glues its eggs to a leaf and carries it like a tray (tool) to the safety of a foam nest. South American cichlids also lay eggs on leaves and will move the eggs on leaves to protected locations. The Sixbar Wrasse, when presented with food pellets too large to swallow, used a rock held in its mouth as a tool to batter the food pellet. Archerfish learn to shoot a stream of water at terrestrial insects above the water. Damselfish clean a vertical rock face by gathering sand in their mouth and sandblasting (Keenleyside 1979). Damselfish also maintain desirable algal patches by weeding out other algal species.
Fish recognize each other, which allows for cooperative behavior, social learning, and signaling (Griffiths and Ward 2011). Fish can recognize familiar individuals by their unique odor or visual cues. They can also identify close kin. Recognition provides fish with the ability to form large shoals of similar fish, thereby creating safety in numbers. Migrating Steelhead Trout, for example, form associations that persist during their long-distance migrations. Constant associations may lead to formation of social networks among individuals (Krause et al. 2017) and enhance social learning pathways. Social learning was previously thought to be restricted to birds and mammals. However, experiments with fish demonstrate numerous situations where individual fish learn from others (Brown and Laland 2011). For example, fish can learn about risky habitats from their own experience or from the reactions of other fish. Human fishing activities may influence fish learning. Removal of more knowledgeable individuals may disrupt social transmission of information, such as location and routes to feeding or spawning grounds. Furthermore, the improved effectiveness of fishing gears may at some point overcome the ability of fish to learn (Ferno et al. 2011), which means fish can no longer adapt their behavior to avoid being caught. Understanding how fish learn has important and unexplored applications, such as training of fish before conservation restocking.
Questions to ponder:
International Association for the Study of Pain (IASP) states that “activity induced in the nociceptor and nociceptive pathways by a noxious stimulus is not pain, which is always a psychological state.” Pain requires a state of consciousness, which is processed in the cortex in humans. Do we know where fish consciousness resides? How do we know fish are aware? Are you convinced that fish can and do experience pain?
5.6 Welfare and Well-Being
The emerging picture informs our understanding of the intelligence, learning, and memory of fish. Evidence that fish are sensitive to pain and are self-aware is sufficient to lead many to conclude that fish exhibit relevant, morally significant capacity to suffer. Animals that are intelligent have greater capacity to suffer, and people are more likely to show empathy toward fish that they believe are intelligent (Bekoff 12014; Brown 2015). Fish are popular pets—only cats and dogs are more popular (Iwama 2007). Fish caught by global fisheries number in the trillions, and fish farming kills billions each year, more than the number of chickens killed for human consumption. Yet, wild fish are hardly as visible to us and do not share a common environment. This separation creates a challenge for questions of animal welfare (Meijboom and Bovenkerk 2013).
The term “welfare” addresses the physical and mental health and well-being of a fish or group of fish. Scientists and ethicists differ on how to approach animal welfare. For example, the animal welfare views held by individuals may be based on
- Function, that is, indicative of growth or fecundity;
- Nature, which relates to the ability to lead a natural life in the wild; or
- Feelings, which focuses on mental states rather than physical health and emphasizes not only the avoidance of stress or fear, but also the opportunity to experience positive feelings (Fraser 1995).
The function-based approach is advocated by recreational angling interests (Arlinghaus et al. 2007). The third view is advocated by animal welfare advocates. Good animal welfare practices mean fish “are healthy and have what they want” (Dawkins 2008). This statement obliges us to determine animals’ wants and presupposes that we can determine positive states of emotion. However, the scientific findings regarding pain and consciousness are now being filtered through ethical disputes between anglers, fishing and fish farming industries, and animal-rights advocates to develop norms and legal protections for fish. As expected, the animal rights advocates stress that the lives of fish are valuable in and of themselves (intrinsic value) and not because of their utility to humans. The views of others who value fish for human uses are in conflict. Therefore, they may question whether it is relevant that fish feel pain and suffer or can feel pleasure and enjoyment.
The views of stakeholders and society at large about mental capacities of fish and their moral status have not been systematically examined, but welfare decisions will have to consider a plurality of moral views. Attempts to provide objective measures of welfare in captivity or during and after capture may not be easily determined from existing models of domestic livestock (McGreevy et al. 2018; Barrell 2019; Browning 2020). While some scientists reject the empirical evidence on fish sentience, animal welfare practices are costly, debatable, and engage numerous social values and novel questions (Jacquet 2018). Only in the context of different fishing practices does it make sense to engage in the debates over animal welfare. Behavioral and physiological assessment of fish can be conducted to determine if fish are relaxed, agitated, anxious, or distressed. For example, levels of cortisol in the blood are universally used to indicate stress, a negative welfare status.
Questions to ponder:
In the future, do you believe that fish will continue to be treated as commodities—that is, caught, farmed, and eaten without moral consideration? What moral status will fish occupy in the future? Which of the three views (feelings, nature, functions) would you adopt to decide how to address welfare of fish?
5.7 Fish as Research Subjects
Fish are used in a wide variety of research studies, and this use may cause suffering or death. Therefore, suffering or death of research animals must be justified by scientific or medical advances that could not be achieved in any other way. Any scientist planning to use animals in their research must first show why there is no alternative, and consider the three Rs in order to minimize numbers of fish suffering:
- Replace the use of animals with alternative techniques or avoid the use of animals altogether.
- Reduce the number of animals used to a minimum, to obtain information from fewer animals or more information from the same number of animals.
- Refine the way experiments are carried out, to make sure animals suffer as little as possible. This includes better housing and improvements to procedures that minimize pain and suffering and/or improve animal welfare.
From a risk-management perspective, the ethical costs of making an error in this judgment are huge given the massive number of fish that are involved in fisheries and scientific research (Brown 2015). Guidelines for the use of fish in research are most often informed by empirical evidence with regard to the capacity of animals to experience pain (Sneddon 2015; Message and Greenhough 2019). Scientific associations have developed ethical justifications for allowable use of fish in research (Metcalfe and Craig, 2011; AFS 2014; Elsevier 2012).
5.8 Fish as Pets
Although welfare of fish as pets has been historically ignored, recent findings on fish pain, aesthetic concerns, and higher costs among serious hobbyists have raised concerns. Fish, such as Goldfish, have feelings and perceive pain and are capable of learning. Pet fish owners who provide adequate environments will see healthy fish that display a broader array of behaviors in fish tanks. Some estimates suggest that the aquarium-keeping industry is worth between 15 and 40 billion U.S. dollars globally, with approximately 10% of the U.S. and U.K. populations already invested in aquarium keeping (Marchio 2018; Sneddon and Wolfenden 2019). Growing numbers of veterinarians are gaining clinical experience with pet fish (Hartman et al. 2006). Common welfare issues include purchasing fish that grow too large for aquariums, overstocking an aquarium, water quality, inadequate water filtration, poor diets, and mixing incompatible species. Many aquarium keepers have misconceptions regarding the lifespans of fish and the required level of care. Further, when individual fish are affordable, their perceived value and concern for welfare are very low. Many unique varieties of Goldfish are prone to medical conditions that affect their welfare in captivity (Brown et al. 2019). Other welfare issues relate to the conditions in the supply chain, which often includes harvesting from wild populations and little concern for welfare during transport. Because fish are often one of the first pets that children obtain and care for, there is great opportunity for education in welfare concerns and conservation via the aquarium-keeping industry (Marchio 2018). In the future, better education, veterinary care, and creating codes of practice should improve the welfare of ornamental fish in captivity (Walster et al. 2015).
5.9 The Angler’s Dilemma
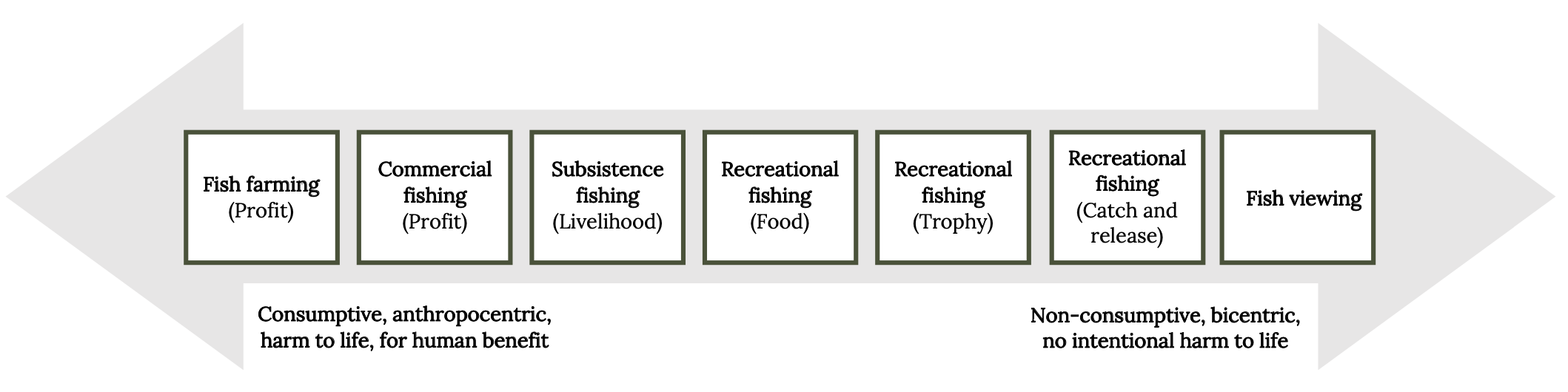
Justification for other uses of fish often considers the type of benefits that humans derive and whether harm is intentional (Figure 5.5). When viewing fish, humans are not consuming or removing individuals and do not intend to harm them. Consequently, little attention is paid to welfare issues associated with viewing wild fish. However, recreational fish may be pursued for food, competition, trophies, or leisure (catch and release). Most recreational anglers practice a mix of these pursuits, which complicates the ethical considerations. Subsistence and commercial fishing and fish farming are responsible for the highest numbers of fish killed worldwide.
The angler’s dilemma about treatment and welfare of the fish captured has a long history. The utilitarian argument maintains that the only morally justifiable reason for catching fish is to kill and eat them. When assessing the consequences of our actions, it is necessary to take the interests of animals seriously and to weigh any adverse effects on those interests from human actions as part of the consequences of those actions (Singer 1975). Consequently, some anglers feel strongly that catching fish for mere sport, not for food, is objectional. British poet and fly angler John Gay (1685–1732) argued in favor of the moral superiority of fly-fishing over other forms of angling on the grounds that fly anglers did not mistreat worms, insects, small fish, and frogs as did bait fishers (Schullery 2008). The first fishing code of ethics that advised anglers on how to minimize cruelty to fish was published in 1876 (Raymond 1876). Despite the long history of concerns, the welfare concerns about recreational fishing are still hotly debated today.
“If a fish could scream, a lot of things would be different”—this statement was attributed to fly-fishing writer Charles Brooks (Schullery 2008). It is easier for us to discount the suffering of fish because they do not make the intensity of their suffering known to us in a way that evokes our emotional response. As such, we would never permit fly-fishing for songbirds. Roderick Haig-Brown, in “The People’s Right to Go Fishing” (1939, 162) wrote, “There can be no doubt that animals, birds and fish feel pain. . . . They feel pain; and they know fear—not fear of death or future suffering—but immediate fear of an immediate, visible threat to themselves, fear of present pain or present restraint, and ever fear of something directly associated with pain or restraint.” Apparently, Haig-Brown was decades ahead in refuting the long-held notion that fish lack the neurological mechanisms to feel pain or experience awareness.
Among the three perspectives on welfare with respect to recreational fishing, most angling interests have argued for the functions-based or feelings-based approaches, and not the nature-based approach. Feelings-based approaches sometimes critique fishing terms, such as “fighting” or “playing” the fish. Writer John McPhee (2002) considered “playing” a euphemism for “at best torturing and at worst killing a creature you may or may not eat.” And de Leeuw (1996) maintained that sportfishing involves (a) killing fish and (b) purposefully inflicting pain and suffering in them in order for anglers to have “sport” with them. This is sometimes referred to as the “sadistic” argument against sportfishing. If one holds true to the principle of avoiding all suffering in animals, then they must reject all sportfishing. Sport anglers value sport with fish more than they respect the lives of animals pursued (de Leeuw 1996). Participation in sportfishing requires justification for inflicting avoidable pain and suffering.
Participants will claim that the utilitarian benefits of sportfishing outweigh any harm to fish. If conservation does not arise from angling, then clearly one cannot justify angling (de Leeuw 1996). Anglers support conservation via license fees, excise taxes, support for conservation organizations, and participation in creel surveys and volunteer work. Do these efforts justify the avoidable pain and suffering? One must consider the activities supported and whether they create more fish in the future. Do these activities outweigh harm to fish? Answering that question is a very substantial task. The argument proposed by de Leeuw (1996) did precipitate other counterarguments (Chipaniuk 1997; List 1997). As outlined by Olsen (2003), the sadistic argument is as follows (note: I replaced “sport fisherman” with the gender-neutral term “angler”):
- Premise: if the angler deliberately inflicts pain on fish and the infliction of pain on fish is the source of enjoyment, then sportfishing is an activity that involves deliberate and excessive cruelty morbidly enjoyed;
- Premise: the angler deliberately inflicts pain on fish;
- Premise: the infliction of pain on fish is the source of enjoyment for anglers;
- Premise: all activities that involve deliberate and excessive cruelty morbidly enjoyed are sadistic;
- Premise: all sadistic activities are unethical activities;
- Conclusion: sportfishing is an unethical activity.
Indigenous people advocate for banning the practice of catch-and-release fishing. In Switzerland and Germany, catch-and-release fishing is considered inhumane and is now banned. In some cases, the acceptance of the pain and suffering argument has led to bans on competitive fishing, put-and-take fishing, and use of live baitfish. The sadistic argument has not persisted because in the mind of the angler, there is a disconnect between fish behavior and fish pain. It is not the infliction of pain in fish that the angler enjoys but the experience of enticing the fish to bite and retrieving the struggling fish. If the fish did not struggle on the line, it is unlikely that sport anglers would pursue fishing. To argue that all who participate in sportfishing are sadists is an attack on the person more than the argument. Argumentum ad hominem, which refers to an attack on the person and not the argument, is a weak form of argumentation. Sportfishing may be wrong, but those who participate in the activity need not be sadists.
Those who argue for welfare considerations for fish from a functions-based view recognize that angling induces stress and may cause injuries (Arlinghaus et al. 2007, 2009; Arlinghaus and Schwab 2011). For example, angling often causes injuries that may depress the ability of the fish to feed and survive after release (Thompson et al. 2018). The pragmatic argument maintains that recreational fishing is a legitimate leisure activity that also contributes to overall food security and personal nutrition (Cooke et al. 2018). Furthermore, fishing may serve as a therapeutic coping mechanism for distressed individuals (Craig et al. 2020). The pragmatic argument may or may not accept the existence of pain, suffering, and consciousness in fish. However, rather than applying a rigid egalitarian perspective that fish morally deserve equal status, the pragmatist adapts to the complexity of real-life tradeoffs (Crittendon 2003; Dawkins 2017). Hence, the focus is on the welfare of fish from measures of health and fitness of individuals and attempt to balance the interests of anglers with the interests of fish. Anglers and fisheries managers may implement regulations or recommendations for gear choice, landing nets, catch-and-release fishing, and other practices that minimize fish welfare impairments (Ferter et al. 2020).
In practice, the weighing of concerns of fish and humans has not been a routine activity (Sandøe et al. 2009), but it is obvious in some fishing codes of ethics. Cooke and Suski (2005) and Cooke and Sneddon (2007) suggested that there are specific actions that anglers could take to minimize negative consequences on fish.
- Minimize angling duration.
- Minimize air exposure and improve handling.
- Terminal tackle choices can affect fish.
- Avoid angling in extreme environmental conditions or habitats.
- Avoid angling during the reproductive period.
- Avoid tethering of live fish on stringers.
- All fish bleeding from hooked gills should be killed.
- Dispatching a caught fish should be undertaken quickly and humanely by a blow to the head or spiking through the brain just behind the eye.
Questions to ponder:
Consider the last time you went fishing for recreation. How did you handle your catch? Was it released? If you kept it, did you kill it in a humane way? Watch this video, “The Right Way to Kill a Fish.” The video demonstrates the use of ikejime for humane killing of recreationally caught tuna. Do you know how the fish you purchase to eat are caught and killed?
5.10 Commercial and Subsistence Fishing
Most discussions around commercial and subsistence fishing focus on conservation and maintenance of traditional fishing-based livelihoods and not on the emerging evidence of pain and suffering of fish. Suffering is caused to wild-caught fish throughout the process of capture until death. Yet, discussion of capture, landing, and killing practices in commercial fisheries is uncommon. However, advocates for animal welfare for commercially caught wild fish highlight the trillions of slow deaths (Mood 2010). Globally, 84 million tonnes of fish were harvested in 2019. In terms of numbers, between 0.8 and 2.3 trillion fish were killed each year by commercial fishing operations between 2007 and 2017 (based on registered landings only, not including all bycatch and discards; fishcount.org.uk). Observations of fishing at sea are difficult; but a few studies report that most fish were alive and conscious when landed and left to die of asphyxia or gutted alive. Death may typically take one hour (trawls), from one to four hours (seines), and from four to six hours (hooks), depending on the species, while nets may take up to 24 hours (Håstein et al, 2005). Moreover, the practice of placing live fish on ice merely prolongs the suffering.
Commercial and subsistence fishing provides food necessary for human sustenance, which would qualify as a reason for certain infringements on the interests of fish. However, the compromises that are morally acceptable depend on the philosophy being applied (Sandøe et al. 2003). If one argues that it is morally impermissible to harvest fish from the wild, and if it were to be prohibited, the lifestyle of many traditional and modern communities would be lost. Perhaps the moral benefit of preserving these communities and lifestyles outweighs the harm of at least certain kinds of fishing. The principle of cultural preservation would claim that fishing is a long-standing cultural practice that is central to a community’s way of life. The cultural preservation arguments would support claims for preserving fishing as a moral consideration to be weighed against other moral considerations. These arguments are especially relevant for small-scale artisanal or subsistence fishing.
Welfare of commercially caught and farmed fish from the wild is the last frontier for animal food production (Cook et al. 2018; Browman et al. 2019). These types of debates are inevitable, and guidelines for responsible fisheries were outlined in the Food and Agricultural Organization Code of Responsible Fisheries (FAO 1995). The FAO has no legislative authority, so the code is voluntary and depends on the willingness of the fishing industry, fishery managers, fishing communities, and peer pressure for adoption. Stakeholders in the commercial and subsistence fishing sectors must participate and raise concerns about the human interests to be balanced against interests of fish (Lam and Pitcher 2012; Lam 2019).
Question to ponder:
The largest fishery in the USA targets Alaska Pollock via midwater trawls. Vessels harvest, process, package, and freeze catch within hours of harvest to produce frozen fillets, fish sticks, and to supply McDonald’s Filet-O-Fish®. Learn more about this large commercial fishery by watching this video. How might you address fish welfare issues in this fishery?
5.11 Welfare Considerations in Fish Farming
Fishing farming is the fastest-growing animal producing sector in the world and plays an important role in global food security. Since the 1990s, most growth in fish production has come from aquaculture, which currently accounts for 49% of total fish production (FAO 2020). Many challenges face the fish farming sector as it grows (Klinger and Naylor 2012), and fish welfare has not been a priority concern. Between 48 and 160 billion farmed fishes were slaughtered in 2015 (fishcount.org.uk). Fish farmers understand the many benefits to improving animal welfare and know that improvements to food production systems that allow fish to thrive, grow, and stay healthy will result in higher-quality fish products. Although there are currently no laws providing protection of farm-raised fish in the United States or in the European Union, the emergence of animal welfare concerns led to criteria for feeding, housing, health, and emotional states for all captive animals, including farmed-fish criteria (Botreau et al. 2007; Levenda 2013). For example, Norway is the world’s leading exporter of salmon and trout, and the Norwegian Animal Welfare Act (passed in 2010, Olesen et al. 2011) protects all vertebrates raised for food. Salmon farming has grown in size and intensity, from net-pen culture to land-based salmon farms, some of which are capable of harvesting over 1,000 tonnes per year (https://salmonbusiness.com/these-are-the-leading-land-based-salmon-farms-in-the-world-right-now/).
Fish farming adopts welfare indicators to judge the state of the welfare of farmed fish. Prominent welfare standards exist for Atlantic Salmon and Rainbow Trout (Noble 2020). Welfare indicators include disease, parasites, wounds, anomalies, and behavior, which are each scored from good to bad. High-intensity, high-output fish farms have the greatest welfare concerns due to overcrowding, handling, transport, starvation, and slaughter (Ashley 2007; Santurtun et al. 2018). A global assessment of welfare of 41 farmed fish indicated that the majority of fish farms have poor welfare conditions (Saraiva et al. 2019).
Indicators of the welfare of fish may be used by fish farms to draw attention to early signs of problems related to captivity conditions and allow intervention before harmful states are reached (Arechavala-Lopez and Saraiva 2019). For example, the social environment for Nile Tilapia had negative effects on stress levels, growth, and aggression, all of which can be resolved with changes in lighting, environment color, and enrichment structures (Gonçalves-de-Freitas et al. 2019). The more intelligent an animal, the more cognitive stimulation it requires to avoid boredom and experience positive states such as pleasure and excitement. Changes in the design of fish farms that recognize the unique behavioral needs of the fish being raised may yield important benefits to fish welfare and farm yields (Fife-Cook and Franks 2019). Furthermore, Norwegian consumers are willing to pay more for improved welfare in farmed salmon (Grimsrud et al. 2013).
Question to ponder:
Watch “Rethink Fish” here. What questions or concerns do you have about how your farmed fish are raised?
5.12 Killing Fish
Fish slaughter is the process of killing fish, typically after harvesting at sea or from fish farms. Despite the trillions of fish slaughtered annually, they are excluded from the U.S. Humane Slaughter Act (P.L. 85-765; 7 U.S.C. 1901 et seq.). This means that fish are killed without regard to the suffering they endure before death.
In 2004, the European Food Safety Authority observed that “Many existing commercial killing methods expose fish to substantial suffering over a prolonged period of time.” The Aquatic Animal Health Code of the World Organisation for Animal Health considers the following slaughter methods inhumane: air asphyxiation, ice bath, salt or ammonia bath, and exsanguination without stunning. More humane killing methods include stunning, pithing, and electrical stunning, and inventors have filed dozens of patents for stunning devices (Lines et al. 2003). Percussive and electric stunning causes loss of consciousness, based on EEG correlations (Robb et al. 2000). While some ethicists have argued that there are no available humane slaughter methods for fish (Browning and Veit 2020), improvements in killing techniques are being adopted by some fisheries (Goldfarb 2019).
Recent discoveries demonstrate that the more humanely a fish is killed, the better it tastes (Bane 2015; Lefevre et al. 2016; Goes et al. 2019). The combination of stress and intense physical activity can increase the degree of protein denaturation, leading to faster muscle softening (Hultmann et al 2012). This discovery provides a utilitarian argument for humane killing. Humane slaughter has been adopted in some fish farms. Are consumers willing to pay? Some high-end restaurants purchase “Humane Harvest” cod for their menus, providing direct value for welfare of sentient animals (Carlier and Treich 2020).
Question to ponder:
Socrates, in Plato’s Republic, said, “Would this habit of eating animals not require that we slaughter animals that we knew as individuals, and in whose eyes we could gaze and see ourselves reflected, only a few hours before our meal?” (360 BC). How often have you looked into the eyes of an animal you were about to slaughter for a meal? Do you agree with Marc Bekoff (2018) that “It’s time to stop pretending that fish don’t feel pain.”
Watch “How to Kill a Fish” here.
If you had to kill an animal in order to eat it, would you eat less meat?
5.13 Closing Thoughts
The debates over pain in fish have illustrated the difficulty that people have in changing long-held views and behaviors. Scientists need to do more than provide evidence in scientific articles that test whether fish are sentient and emotional beings who feel pain. Dialogue about the issue has more frequently been presented at one-way arguments that were certain to be countered with alternative interpretations. Simply giving people more information does not necessarily change how people feel about an issue. This is referred to as the information deficit model, which attributes skepticism or hostility to a lack of understanding and a lack of information. Scientists who study the public understanding of science have concluded the information deficit model is an insufficient strategy for communication and changing people’s views. One alternative strategy for communicating in contentious situations involves making personal connections in ways that permit the participants to listen, share, and connect with others in order to understand the mental model(s) used by others (Crandall et al. 2020). The process of dialogue can build understanding of personal values, interests, ideology, worldviews, moral foundations, group identity, and religious background that contribute to disputes. Although disagreements will continue, the process permits all stakeholders in fish welfare issues to contribute to solutions.
Profile in Fish Conservation: Culum Brown, PhD

Culum Brown is Professor of Fish Behavioral Ecology at Macquarie University, where he directs research in the Behaviour, Ecology and Evolution Laboratory. His lab studies social learning and memory in a variety of fish. Some journalists refer to him as Dr. Fish Feelings in recognition of his expertise in fish feeling.
His research has revealed that many fish are sophisticated learners that can retain memories for months. His findings related to social learning in fish have direct implications for conservation and restoration of exploited fish. For example, if older, more experienced fish are preferentially harvested, the collective information on feeding and breeding grounds and migration routes may be lost, thereby reducing growth and survival. Also, widespread use of hatchery-reared fish is inefficient because of the high mortality they experience immediately after release. He developed protocols for life-skills training to improve performance of salmonids after release in the wild. Expanding our knowledge of the role of learning in fish behavior has direct applications to welfare of fish raised in captivity for release or human food. Understanding the behavioral preferences provides fish farmers with specific ways to enrich the environment.
Dr. Brown’s research asks basic questions about learning and memory in the natural environment. Fish have a richer visual and acoustic environment than humans can appreciate. Fish have advanced sensory capabilities for vision, hearing, and smell, that directly influence their abilities to learn about their environments and communicate with other fish. For example, most fish have four types of cones in their eyes, and therefore they see more colors and see them more vividly than humans can. The ability of some fish to detect polarized and ultraviolet light waves permits them to distinguish more from their environment than humans can see. In addition to vision, fish hear an amazing chorus from animals underwater and communicate with other fish by making all sorts of fishy sounds. Vision, smell, and hearing enable fish to orient in familiar locations and remember locations of food patches, shelter, and breeding sites.
Another character trait explored by Culum Brown’s lab is the notion of personality in fish. His lab has found that personality, laterality, and stress reactivity are all linked. Most humans are right-handed, and other vertebrates show lateral preferences in the brain that translate into sidedness. This question of left-right dominance was seldom studied in fish, until Culum Brown’s lab investigated whether native rainbow fish used one eye or both eyes while looking out for potential dangers. The rainbow fish showed differences in boldness, a personality trait, and their personality was linked to whether one eye or both eyes were dominant.
One of Dr. Brown’s popular research subjects is the Port Jackson Shark, which he calls the “puppies of the sea.” His research discovered the complex social structure and intelligence in the Port Jackson Shark, disputing the notion that sharks are robot-like, antisocial killers. Recent research reveals that Port Jackson Sharks show individual preferences for either left-eye or right-eye dominance, have personalities, and vary in how they respond to handling (docility). Following highly mobile sharks and rays, his research has demonstrated group formation and affiliation among social networks. The abilities to learn, remember, communicate, form relationships, and use tools are all characteristics of sentience.
Brown’s collective works in behavior and cognition have contributed to the formation of a new field of neuroethics of nonhuman animals. He released a collection of works in two books, entitled Fish Cognition and Behaviour, published in 2006 and 2011, and he has published more than 150 scientific articles on fish behavior. He is also Editor of the Journal of Fish Biology. His work on fish cognition is increasingly used as a basis for the justification of positive welfare for fish.
For more information, see https://www.thefishlab.com/PI.html.
Key Takeaways
- Humans use fish in a variety of ways, which may influence how they perceive the value of a fish’s life.
- Fish feel pain and suffer as a consequence, and we must carefully examine welfare, use, and fishing practices.
- Studies of pain in fish examined pain receptors, nerve activity, and behavior change.
- Whether an animal is sentient is based on five capabilities that have been studied scientifically.
- Scientists apply the three Rs—Replacement, Reduction, and Refinement—for consideration when minimizing pain and suffering in experiments.
- Actions to improve welfare in recreational and commercial fisheries and fish farms are part of lively debates.
This chapter was reviewed by Culum Brown.
URLs
Video 1: https://www.youtube.com/watch?v=TS4AM9mPX-8
Video 2: https://www.youtube.com/watch?v=WXCzpamTvcc
Video 3: https://www.ciwf.org.uk/our-campaigns/rethink-fish/
Humane Harvest: https://www.hsa.org.uk/
Video 4: https://www.youtube.com/watch?v=TS4AM9mPX-8
Long Descriptions
Figure 5.1: Anti-fishing slogans “don’t let your kids become hookers,” “fishing hurts,” “Your daddy kills animals” rose in late 1980s and again from 2000-2010 and 2018 and on. Jump back to Figure 5.1.
Figure 5.2: Position of polymodal mechanoreceptors (or nociceptors), mechanothermal receptors, and mechanochemical receptors on the head and face of the rainbow trout. Pale yellow circles: polymodal nociceptor. Black circles: mechanothermal nociceptor. Green circles: mechanochemical receptor. Jump back to Figure 5.2.
Figure 5.3: These are the 5 factors that contribute to sentience in fish: 1. Evaluation behavior of others; form relationships, 2. Remember own actions’ use memory to inform future behavior, 3 . Assess risks and benefits to make decisions, 4. Positive or negative affective states such as pain, fear, pleasure, 5. Some degree or awareness. Jump back to Figure 5.3.
Figure 5.4: Line graph A) The binary model shows that canines, felines, most birds, fish, monkeys, and most other species have no self-awareness. Line graph B) The gradualist view shows a linear climb of self-awareness starting with smaller-brained animals, dogs, cats, pigs, monkeys, parrots, cleaner fish, elephants, dolphins, magpies, and doesn’t reach mirror self-recognition until Hominids. Jump back to Figure 5.4.
Figure 5.5: An arrow displays two categories with (left) consumptive, anthropocentric, harm to life, for human benefit and (right) non-consumptive, bicentric, no intentional harm to life. From left, motivations listed include, 1) fish farming (profit); 2) commercial fishing (profit); 3) subsistence fishing (livelihood); 4) recreational fishing (food); 5) recreational fishing (trophy); 6) recreational fishing (catch and release); 7) fish viewing. Jump back to Figure 5.5.
Figure References
Figure 5.1: Frequency of appearance of “pain in fish” in books since 1965 coincides with appearance of antifishing slogans after 1996. Kindred Grey. 2022. CC BY 4.0. Data from Google ngram viewer.
Figure 5.2: Sketch of Rainbow Trout with locations of nociceptors. Kindred Grey. 2022. Adapted under fair use from Do Fishes Have Nociceptors? Evidence for the Evolution of a Vertebrate Sensory System, by Lynne U Sneddon, Victoria A Braithwaite, and Michael J Gentle, 2003 (doi: 10.1098/rspb.2003.2349). Includes PSM V47 D194 Rainbow Trout Adult Salmo Mykiss Walbaum, by unknown author, 1895 (public domain, https://commons.wikimedia.org/wiki/File:PSM_V47_D194_Rainbow_trout_adult_salmo_mykiss_walbaum.jpg).
Figure 5.3: Diagrammatic representation of the five capabilities that make an animal sentient. Kindred Grey. 2022. Adapted under fair use from “Mental Capacities of Fishes,” by Lynne U. Sneddon and Culum Brown, 2020 (https://doi.org/10.1007/978-3-030-31011-0_4).
Figure 5.4: Two different perspectives on the evolution of self-awareness. Kindred Grey. 2022. CC BY 4.0. Adapted from “Fish, Mirrors, and a Gradualist Perspective on Self-Awareness,” by Frans B. M. de Waal, 2019 (CC BY 4.0, DOI:10.1371/journal.pbio.3000112).
Figure 5.5: Human motivations for types of fish and fish viewing. Kindred Grey. 2022. Adapted under fair use from Tourism and Animal Ethics, by David A. Fennell, 2012, 182 (ISBN 9781138081345).
Figure 5.6: Culum Brown, PhD. Used with permission from Culum Brown. CC BY 4.0.
Text References
Adriaense, J. E. C., S.E . Koski, L. Huber, and C. Lamm. 2020. Challenges in the comparative study of empathy and related phenomena in animals. Neuroscience & Biobehavioral Reviews 112:62–82.
AFS. 2014. Guidelines for the use of fishes in research. Use of Fishes in Research Committee (joint committee of the American Fisheries Society, the American Institute of Fishery Research Biologists, and the American Society of Ichthyologists and Herpetologists). American Fisheries Society, Bethesda, MD.
Agetsuma, M., H. Aizawa, T. Aoki, R. Nakayama, M. Takahoko, M. Goto, T. Sassa, K. Kawakami, and H. Okamoto. 2010. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nature Neuroscience 13:1354–1356.
Allen, C. 2013. Ethics, law, and the science of fish welfare. Between the Species 16:68–85.
Appleby, M. C., and P. Sandøe. 2002. Philosophical debate on the nature of well-being: implications for animal welfare. Animal Welfare 11:283–294.
Ari, C., and D. P. D’Agostino. 2016. Contingency checking and self-directed behaviors in Giant Manta Rays: do elasmobranchs have self-awareness? Journal of Ethology 34:167–174.
Arlinghaus, R., S. J. Cooke, A. Schwab, and I. G. Cowx. 2007. Fish welfare: a challenge to the feelings-based approach, with implications for recreational fishing. Fish and Fisheries 8(1):57–71.
Arlinghaus, R., and A. Schwab. 2011. Five ethical challenges to recreational fishing: what they are and what they mean. American Fisheries Society Symposium 75:219–234.
Arlinghaus, R., A. Schwab, S. Cooke, and I. G. Cowx. 2009. Contrasting pragmatic and suffering-centred approaches to fish welfare in recreational angling. Journal of Fish Biology 75:2448–2463.
Ashley, P. J. 2007. Fish welfare: current issues in aquaculture. Applied Animal Behaviour Science 104:199–235.
Ashley, P. J., S. Ringrose, K. L. Edwards, E. Wallington, C. R. McCrohan, and L. U. Sneddon. 2009. Effect of noxious stimulation upon antipredator responses and dominance status in Rainbow Trout. Animal Behaviour 77: 403–410. https://doi.org/10.1016/j.anbehav.2008.10.015.
Ashley, P. J., L. U. Sneddon, and C. R. McCrohan. 2007. Nociception in fish: stimulus-response properties of receptors on the head of trout Oncorhynchus mykiss. Brain Research 1166:47–54. DOI: 10.1016/j.brainres.2007.07.011.
Bane, B. 2015. The more humanely a fish is killed, the better it tastes. Science. doi:10.1126/science.aad7558.
Barrell, G. K. 2019. An appraisal of methods for measuring welfare. Frontiers in Veterinary Science 6:289. https://doi.org/10.3389/fvets.2019.00289.
Bekoff, M. 2014. Rewilding our hearts: building pathways of compassion and coexistence. New World Library, Novato, CA.
Bekoff, M. 2018. It’s time to stop pretending fishes don’t feel pain. Psychology Today (January 7). Available at https://www.psychologytoday.com/us/blog/animal-emotions/201801/its-time-stop-pretending-fishes-dont-feel-pain. Accessed July 28, 2020.
Bernardi, G. 1912. The use of tools by wrasses (Labridae). Coral Reef 31:39. doi:10.1007/s00338-011-0823-6.
Beukema, J. J. 1969. Angling experiments with carp. Netherlands Journal of Zoology 20:81–92.
Botreau, R., I. Veissier, A. Butterworth, M. B. M. Bracke, and L. J. Keeling. 2007. Definition of criteria for overall assessment of animal welfare. Animal Welfare 16:225–228.
Bovenkerk, B., and F. J. B. Meijboom. 2012. The moral status of fish: the importance and limitations of a fundamental discussion for practical ethical questions in fish farming. Journal of Agricultural and Environmental Ethics 25:843–860.
Braithwaite, V. 2010. Do fish feel pain? Oxford University Press.
Broglio, C., F. Rodriguez, and C. Salas. 2003. Spatial cognition and its neural basis in teleost fishes. Fish and Fisheries 4:247–255.
Broom, D. M. 2014. Sentience and animal welfare. CABI, Boston, MA.
Browman, H. I., S. J. Cooke, I. G Cowx, S. W. G. Derbyshire, A. Kasumyan, B. Key, J. D. Rose, A. Schwab, A. B. Skiftesvik, E. D. Stevens, C. A. Watson, and R. Arlinghaus. 2019. Welfare of aquatic animals: where things are, where they are going, and what it means for research, aquaculture, recreational angling, and commercial fishing. ICES Journal of Marine Science 76:82–92.
Browman, H. I., and A. B. Skiftesvik. 2011. Welfare in aquatic organisms: is there some faith- based HARKing going on here? Diseases of Aquatic Organisms 94:255–257.
Brown, C. 2001. Familiarity with the test environment improves escape responses in the Crimson Spotted Rainbowfish, Melanotaenia duboulayi. Animal Cognition 4:109–113.
Brown, C. 2012. Tool use in fishes. Fish and Fisheries 13:105–115.
Brown, C. 2015. Fish intelligence, sentience and ethics. Animal Cognition 18:1–17.
Brown, C. 2016. Comparative evolutionary approach to pain perception in fishes. Animal Sentience 3(5). DOI: 10.51291/2377-7478.1029.
Brown, C. 2017. A risk assessment and phylogenetic approach. Animal Sentience 16(3). DOI: 10.51291/2377-7478.1219.
Brown, C., and C. Dorey. 2019. Pain and emotion in fishes: fish welfare implications for fisheries and aquaculture. Animal Studies Journal 8(2):175–201.
Brown, C., J. Krause, and K. Laland. 2011. Fish cognition and behavior. 2nd ed. Blackwell, Oxford.
Brown, C., and K. Laland. 2003. Social learning in fishes: a review. Fish and Fisheries 4:280–288.
Brown C., and K. Laland. 2011. Social learning in fishes. Pages 240–257 in C. Brown, J. Krause, and K. Laland, editors, Fish cognition and behavior, 2nd ed, Blackwell, Oxford.
Brown, C., K. Laland, and J. Krause. 2011. Fish cognition and behavior. Pages 1–9 in C. Brown, J. Krause, and K. Laland, editors, Fish cognition and behavior, 2nd ed., Blackwell, Oxford.
Brown, C., and K. Warburton. 1999. Differences in timidity and escape responses between predator-naïve and predator-sympatric rainbowfish populations. Ethology 105:491–502.
Brown, C., D. Wolfenden, and L. Sneddon. 2019. Goldfish (Carassius auratus). Pages 467–478 in J. Yeates, editor, Companion animal care and welfare: the UFAW companion animal handbook, John Wiley & Sons, Somerset, NJ.
Browning, H. 2020. Assessing measures of animal welfare. [Preprint] URL: http://philsci-archive.pitt.edu/id/eprint/17144. Accessed June 15, 2020.
Bshary, R., and C. Brown. 2014. Fish cognition. Current Biology 24(19):R947–R950.
Bshary R., S. Gingins, and A. L. Vail. 2014. Social cognition in fishes. Trends in Cognitive Science 18:465–471.
Bshary R., A. Hohner, K. Ait-el-Djoudi, and H. Fricke. 2006. Interspecific communicative and coordinated hunting between grouper and Giant Moray Eels in the Red Sea. PLoS Biology 4(12):e431. doi:10.1371/journal.pbio.0040431.
Bshary, R., W. Wickler, and H. Fricke. 2002. Fish cognition: a primate’s eye view. Animal Cognition 5:1–13.
Burghardt, G. M. 2015. Play in fishes, frogs and reptiles. Current Biology 25:R9–R10.
Burghardt, G. M., V. Dinets, and J. B. Murphy. 2015. Highly repetitive object play in a cichlid fish (Tropheus duboisi). Ethology 121:38–44.
Carlier, A., and N. Treich. 2020. Directly valuing animal welfare in (environmental) economics. International Review of Environmental and Resource Economics 14:113–152.
Cerqueira, M., S. Millot, M. F. Castanheira, A. S. Félix, T. Silva, G. A. Oliveira, C. C. Oliveira, C. I. M. Martins, and R. F. Oliveira. 2017. Cognitive appraisal of environmental stimuli induces emotion-like states in fish. Scientific Reports 7:13181. https://doi.org/10.1038/s41598-017-13173-x.
Chipeniuk, R., 1997. On contemplating the interests of fish. Environmental Ethics 19(3):331–332.
Clegg, I. L. K. 2018. Cognitive bias in zoo animals: an optimistic outlook for welfare assessment. Animals 8:104. doi: 10.3390/ani8070104.
Cook, K. V., A. J. Reid, D. A. Patterson, K. A. Robinson, J. M. Chapman, S. G. Hinch, and S. J. Cooke. 2018. A synthesis to understand responses to capture stressors among fish discarded from commercial fisheries and options for mitigating their severity. Fish and Fisheries 20:25–43.
Cooke, S. J., and L. U. Sneddon. 2007. Animal welfare perspectives on recreational angling. Applied Animal Behaviour Science 104:176–198.
Cooke, S. J., W. M. Twardek, R. J. Lennox, A. J. Zoldero, S. D. Bower, L. F. G. Gutowsky, A. J. Danylchuk, R. Arlinghaus, and D. Beard. 2018. The nexus of fun and nutrition: recreational fishing is also about food. Fish and Fisheries 19:201–224.
Coyer, J. A. 1995. Use of a rock as an anvil for breaking scallops by the Yellowhead Wrasse, Halichoeres garnoti (Labridae). Bulletin of Marine Science 57:548–549.
Craig, P. J., D. M. Alger, J. L. Bennett, and T. P. Martin. 2020. The transformative nature of fly-fishing for veterans and military personnel with posttraumatic stress disorder. Therapeutic Recreation Journal 54:150–172.
Crandall, C. A., M. C. Monroe, and K. Lorenzen. 2020. Why won’t they listen to us? Communicating science in contentious situations. Fisheries 45(1):42–45.
Crittenden, C. 2003. Pluhar’s perfectionism: a critique of her (un)egalitarian ethic. Between the Species 13:3.
Darwin, C. 1872. The expression of the emotions in man and animals. John Murray, London.
Dawkins, M. S. 1980. Animal suffering: the science of animal welfare. Dordrecht, Netherlands: Springer.
Dawkins, M. S. 2008. The science of animal suffering. Ethology 114:937–945. doi: 10.1111/j.1439-0310.2008.01557.x.
Dawkins, M. S. 2017. Animal welfare with and without consciousness. Journal of Zoology 301:1–10.
de Leeuw, A. D. 1996. Contemplating the interests of fish: the angler’s challenge. Environmental Ethics 18:373–390.
de Waal, F. B. M. 2019. Fish, mirrors, and a gradualist perspective on self-awareness. PLOS Biology 17(2):e3000112. https://doi.org/10. 1371/journal.pbio.3000112.
Diggles, B., and H. I. Browman. 2018. Denialism and muddying the water or organized skepticism and clarity? THAT is the question. Animal Sentience 21(10).
Dinets, V. 2016. No cortex, no cry. Animal Sentience 3(7): DOI: 10.51291/2377-7478.1027.
Dzieweczynski, T. L., and O. L. Hebert. 2012. Fluoxetine alters behavioral consistency of aggression and courtship in male Siamese Fighting Fish, Betta splendens. Physiology and Behavior 107:92–97.
Elder, M. 2018. Fishing for trouble: the ethics of recreational angling. Pages 277–301 in A. Linzey and C. Linzey, editors, The Palgrave handbook of practical animal ethics. The Palgrave Macmillan Animal Ethics Series. Palgrave Macmillan, London.
Elsevier. 2012. Guidelines for the treatment of animals in behavioural research and teaching. Animal Behaviour 83 301–309. doi:10.1016/j.anbehav.2011.10.031.
FAO. 1995. Code of Conduct for Responsible Fisheries. Food and Agriculture Organization of the United Nations, Rome.
FAO. 2020. The state of the world fisheries and aquaculture 2020: sustainability in action. Food and Agriculture Organization of the United Nations, Rome. Available at: http://www.fao.org/documents/card/en/c/ca9229en.
Fennell, D. A. 2012. Tourism and animal ethics. Routledge, London and New York.
Ferno, 2011. Fish behaviour, learning, aquaculture and fisheries. Pages 359–404 in C. Brown, K. Laland, and J. Krause, editors, Fish cognition and behavior, 2nd ed.. Blackwell, Oxford.
Ferter, K., S. J. Cooke, O-B. Humborstad, J. Nilsson, and R. Arlinghaus. 2020. Fish welfare in recreational fishing. Pages 463–485 in A. Fernö, A. Pavlidis, J. W. van de Vis, and T. S. Kristiansen, editors, The Welfare of Fish (Animal Welfare 20). Springer.
Fife-Cook, I., and B. Franks. 2019. Positive welfare for fishes: rationale and areas for future study. Fishes 4:31.
Fraser D. 1995. Science, values and animal welfare: exploring the inextricable connection. Animal Welfare 4:103–117.
Goes, E. S. R., M. D. Goes, P. L. de Castro, J. A. Ferreira de Lara, A. C. P. Vital, and R. R Ribeiro. 2019. Imbalance of the redox system and quality of tilapia fillets subjected to pre-slaughter stress. PLoS ONE 14(1):e0210742. https://doi.org/10.1371/journal.pone.0210742.
Goldfarb, B. 2019. How should we treat fish before they end up on our plates? High Country News, March 20, 2019. Available at: https://www.hcn.org/issues/51.6/fish-how-should-we-treat-fish-before-they-end-up-on-our-plates. Accessed August 6, 2020.
Gonçalves-de-Freitas, E., M. C. Bolognesi, A. C. dos Santos Gauy, M. L. Brandão, P. C. Giaquinto, and M. Fernandes-Castilho. 2019. Social behavior and welfare in Nile Tilapia. Fishes 4:23.
Gregory, N. 1999. Do fish feel pain? Australian and New Zealand Council of Animal Care Research and Teaching News 12:1–12.
Griffiths, S. W., and A. Ward. 2011. Social recognition of conspecifics. Pages 186–206 in C. Brown, K. Laland, and J. Krause, editors, Fish cognition and behavior, 2nd ed., Blackwell, Oxford.
Grillner, S., and B. Robertson. 2016. The basal ganglia over 500 million years. Current Biology 26:R1088–R1100.
Grimsrud, K. M., H. M. Nielsen, S. Navrud, and I. Olesen. 2013. Households’ willingness-to-pay for improved fish welfare in breeding programs for farmed Atlantic Salmon. Aquaculture 372–375:19–27.
Grosenick, L., T. S. Clement, and R. D. Fernald. 2007. Fish can infer social rank by observation alone. Nature 445:429–432.
Håstein, T., A. D. Scarfe, and V. L. Lund. 2005. Science-based assessment of welfare: aquatic animals. Revue scientifique et technique (International Office of Epizootics) 24(2):529–547.
Horta, O. 2018. Moral considerability and the argument from relevance. Journal of Agricultural and Environmental Ethics 31:369–388.
Hultmann L., T. M. Phu, T. Tobiassen, Ø. Aas-Hansen, and T. Rustad. 2012. Effects of pre-slaughter stress on proteolytic enzyme activities and muscle quality of farmed Atlantic Cod (Gadus morhua). Food Chemistry 134:1399–1408.
Huntingford, F. A., C. Adams, V. A. Braithwaite, S. Kadri, T. G. Pottinger, P. Sandøe, and J. F. Turnbull. 2006. Current issues in fish welfare. Journal of Fish Biology 68:332–372.
IASP. 2019. IASP terminology: pain terms. International Association for the Study of Pain, Washington, DC:. Available at https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698. Accessed 15 June 2020.
Iwama, G. K. 2007. The welfare of fish. Diseases of Aquatic Organisms 75:155–158.
Jacquet, J. 2018. Defining denial and sentient seafood. Animal Sentience 21(8). DOI: 10.51291/2377-7478.1334.
Jones, A. M., C. Brown, and S. Gardner. 2011. Tool use in the Tuskfish Choerodon schoenleinii? Coral Reefs 30:865.
Keenleyside, M. H. A. 1979. Diversity and adaptation in fish behaviour. Springer-Verlag, Berlin.
Keenleyside, M. H. A., and C. E. Prince. 1976. Spawning-site selection in relation to parental care of eggs in Aequidens paraguayensis (Pisces: Cichlidae). Canadian Journal of Zoology 54:2135–2139. doi:10.1139/z76-247.
Key, B. 2015. Fish do not feel pain and its implications for understanding phenomenal consciousness. Biology and Philosophy 30:149–165.
Key, B. 2016a. Why fish do not feel pain. Animal Sentience 3(1). DOI: 10.51291/2377-7478.1011.
Key, B. 2016b. Falsifying the null hypothesis that “fish do not feel pain.” Animal Sentience 3(39). DOI: 10.51291/2377-7478.1070.
Killen, S. S., S. Marras, and D. J. McKenzie.2011. Fuel, fasting, fear: routine metabolic rate and food deprivation exert synergistic effects on risk-taking in individual juvenile European Sea Bass. Journal of Animal Ecology 80(5):1024–1033.
Klefoth, T., C. Skov, A. Kuparinen, and R. Arlinghaus. 2017. Toward a mechanistic understanding of vulnerability to hook-and-line fishing: boldness as the basic target of angling-induced selection. Evolutionary Applications 10:994–1006.
Klinger, D., and R. Naylor. 2012. Searching for solutions in aquaculture: charting a sustainable course. Annual Review of Environment and Resources 37:247–276.
Kohda, M., T. Hotta, T. Takeyama, S. Awata, H. Tanaka, J. Asai, and A. L. Jordan. 2019. If a fish can pass the mark test, what are the implications for consciousness and self-awareness testing in animals? PLOS Biology 17(2):e3000021. https://doi.org/10.1371/journal.pbio.3000021.
Krause, S., A. D. M. Wilson, I. W. Ramnarine, J. E. Herbert-Read, R. J. G. Clément, and J. Krause. 2017. Guppies occupy consistent positions in social networks: mechanisms and consequences. Behavioral Ecology 28:429–438.
Lam, M. E. 2019. Seafood ethics: reconciling human well-being with fish welfare. Pages 177–197 in B. Fischer, editor, The Routledge handbook of animal ethics. Routledge, New York.
Lam, M. E., and T. J. Pitcher. 2012. The ethical dimensions of fisheries. Current Opinion in Environmental Sustainability 4:364–373.
Laubu, C., P. Louâpre, and F-X. Dechaume-Moncharmont. 2019. Pair-bonding influences affective state in a monogamous fish species. Proceedings of the Royal Society B: Biological Sciences 286(1904):20190760.
Lefevre, F., I. Cos, T. G. Pottinger, and J. Bugeon. 2016. Selection for stress responsiveness and slaughter stress affect flesh quality in pan-size Rainbow Trout, Oncorhynchus mykiss. Aquaculture 464:654–664.
Levenda, K. 2013. Legislation to protect the welfare of fish. Animal Law 20:119–144.
List, C. J. 1997. On angling as an act of cruelty. Environmental Ethics 19:333–334.
Lopez-Luna, J., Q. Al-Jubouri, W. Al-Nuaimy, amd L. U. Sneddon. 2017. Activity reduced by noxious chemical stimulation is ameliorated by immersion in analgesic drugs in zebrafish. Journal of Experimental Biology 220:1451–1458. doi:10.1242/jeb.146969.
Lorimer, J. 2007. Nonhuman charisma. Environment and Planning D: Society and Space 25:911–932.
Lucon-Xiccato, T., and A. Bisazza. 2017. Individual differences in cognition among teleost fishes. Behavioural Processes 141:184–195.
Lund, V., C. M. Mejdell, H. Röcklingsberg, R. Anthony, and T. Håstein. 2007. Expanding the moral circle: farmed fish as objects of moral concern. Diseases of Aquatic Organisms 75:109–118.
Marchio, E. A. 2018. The art of aquarium keeping communicates science and conservation. Frontiers in Communication 3:17. doi: 10.3389/fcomm.2018.00017.
Matthews, G., and W. O. Wickelgren. 1978 Trigeminal sensory neurons of the Sea Lamprey. Journal of Comparative Physiology A 123, 329–333. doi:10.1007/BF00656966.
McGreevy, P., J. Berger, N. de Brauwere, O. Doherty, A. Harrison, J. Fiedler, C. Jones, S. McDonnell, A. McLean, L. Nakonechny, C. Nicol, L. Preshaw, P. Thomson, V. Tzioumis, J. Webster, S. Wolfensohn, J. Yeates, and B. Jones. 2018. Using the Five Domains Model to assess the adverse impacts of husbandry, veterinary, and equitation interventions on horse welfare. Animals 8(3):41. doi: 10.3390/ani8030041.
McPhee, J. 2002. The founding fish. Farrar, Straus and Giroux, New York.
Meijboom, F. L. B., and B. Bovenkerk. 2013. Fish welfare: challenge for science and ethics—why fish makes the difference. Journal of Agricultural and Environmental Ethics 26:1–6.
Message, R., and B. Greenhough. 2019. “But it’s just a fish”: understanding the challenges of applying the 3Rs in laboratory aquariums in the UK. Animals 9(12):1075.
Metcalfe, J. D., and J. F. Craig. 2011. Ethical justification for the use and treatment of fishes in research: an update. Journal of Fish Biology 78:393–394.
Michaelson, E., and A. Reisner. 2018. Ethics for fish. Pages 189–206 in A. Barnhill, M. Budolfson, and T. Dogett, editors, Oxford handbook of food ethics.
Michel, M. 2019. Fish and microchips: on fish pain and multiple realization. Philosophical Studies 176:2411–2428.
Millot, S., M. Cerqueira, M. F. Castanheira, Ø. Øverli, C. I. M. Martins, and R. F. Oliveira. 2014. Use of conditioned place preference/avoidance tests to assess affective states of fish. Applied Animal Behaviour Science 154:104–111.
Mood, A. 2010. Worse things happen at sea: the welfare of wild-caught fish. Report by FishCount.org. Available at http://www.fishcount.org.uk/published/standard/fishcountfullrptSR.pdf.
Mood, A., and P. Brooke. 2010. Estimating the number of fish caught in global fishing each year. Available from http://fishcount.org.uk/studydatascreens/frontpage.php.
Ng, Y-K. 2016. Could fish feel pain? A wider perspective. (Ng commentary on Key’s Why fish do not feel pain). Animal Sentience 19:1–3.
Noble, C., K. Gismervik, M. H. Iversen, J. Kolarevic, J. Nilsson, L. H. Stien, and J. F. Turnbull, editors. 2020. Welfare indicators for farmed Rainbow Trout: tools for assessing fish welfare. Fishwell Handbooks. Tromso, Norway.
Olden, J. D., J. R. S. Vitule, J. Cucherousset, and M. J. Kennard. 2020. There’s more to fish than just food: exploring the diverse ways that fish contribute to human society. Fisheries 45(9):453–464.
Olesen, I., A. I. Myhr, and G. R. Rosendal. 2011. Sustainable aquaculture: are we getting there? Ethical perspectives on salmon farming. Journal of Agricultural and Environmental Ethics 24:381–408.
Olsen, L. 2003. Contemplating the intentions of anglers: the ethicist’s challenge. Environmental Ethics 25:267–277.
Paśko, Ł. 2010. Tool-like behavior in the Sixbar Wrasse, Thalassoma hardwicke (Bennett, 1830). Zoo Biology 29:767–773.
Patton, B. W., and V. A. Braithwaite. 2015. Changing tides: ecological and historical perspectives on fish cognition. WIREs Cognitive Science 6:159–176. doi: 10.1002/wcs.1337.
Pittman J. T., and C. S. Lott. 2014. Startle response memory and hippocampal changes in adult zebrafish pharmacologically-induced to exhibit anxiety/depression-like behaviors. Physiology & Behavior 123:174–179.
Plotnik, J. M., F. B. M. de Waal, and D. Reiss. 2006. Self-recognition in an Asian elephant. Proceedings of the National Academy of Sciences USA 103:17053–17057.
Pouca, C. V., and C. Brown. 2017. Contemporary topics in fish cognition and behaviour. Current Opinion in Behavioural Sciences 16:46–52.
Prior, H., A. Schwarz, and O. Güntürkün. 2008. Mirror-induced behavior in the Magpie (Pica pica): evidence of self-recognition. PLoS Biology 6(8):e202. doi:10.1371/journal.pbio.0060202.
Raja, S. N., D. B. Carr, M. Cohen, N. B. Finnerup, H. Flor, S. Gibson, F. J. Keefe, J. S. Mogil, M. Ringkamp, K. A. Sluka, X-J. Song, B. Stevens, M. D. Sullivan, P. R. Tutelman, T. Ushida, and K. Vader. 2020. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. PAIN 161:1976–1982.
Regan, T. 1983. The case for animal rights. University of California Press, Berkeley.
Reilly, S. C, J. P. Quinn, A. R. Cossins, and L. U. Sneddon. 2008. Behavioural analysis of a nociceptive event in fish: comparisons between three species demonstrate specific responses. Applied Animal Behaviour Science 114:248–259. doi:10.1016/j.applanim.2008.01.016.
Reiss, D. 2012. The dolphin in the mirror: exploring dolphin minds and saving dolphin lives. Houghton Mifflin Harcourt, New York.
Robb, D. H. F., S. B. Wotton, J. L. Mckinstry, N. K. Sørensen, and S. C. Kestin. 2000. Commercial slaughter methods used on Atlantic Salmon: determination of the onset of brain failure by electroencephalography. Veterinary Record 147:298–303.
Rose, J. D. 2002. The neurobehavioral nature of fishes and the question of awareness and pain. Reviews in Fisheries Science 10:1–38.
Rose, J. D., R. Arlinghaus, S. J. Cooke, B. K. Diggles, W. Sawynok, E. D. Stevens, and C. D. L. Wynne. 2014. Can fish really feel pain? Fish & Fisheries 15:97–133.
Sandøe, P., S. B. Christiansen, and M. C. Appleby. 2003. Farm animal welfare: the interaction of ethical questions and animal welfare science. Animal Welfare 12:469–478.
Sandøe, P., C. Gamborg, S. Kadri, and K. Millar. 2009. Balancing the needs and preferences of humans against the concerns for fish: how to handle the emerging ethical discussions regarding capture fisheries. Journal of Fish Biology 75:2868–2871.
Santurtun, E., D. M. Broom, and C. J. C. Phillips. 2018. A review of factors affecting the welfare of Atlantic Salmon (Salmo salar). Animal Welfare 27:193–204.
Saraiva, J. L., and P. Arechavala-Lopez. 2019. Welfare of fish—no longer the elephant in the room. Fishes 4(3):39.
Saraiva, J. L., P. Arechavala-Lopez, M. F. Castanheira, J. Volstorf, and B. A. Heinzpeter Studer. 2019. Global assessment of welfare in farmed fishes: the FishEthoBase. Fishes 4(2):30.
Schnitzler, A., and M. Ploner. 2000. Neurophysiology and functional neuroanatomy of pain perception. Journal of Clinical Neurophysiology 17:592–603. doi: 10.1097/00004691-200011000-00005
Schullery, P. 2008. If fish could scream: an angler’s search for the future of fly fishing. Stackpole Books, Mechanicsburg, PA.
Schuster, S. 2007. Quick guide: archerfish. Current Biology 17:R494–R495.
Singer, P. 1975. Animal liberation: a new ethics for our treatment of animals. Random House, New York.
Singer, P. 2010. Fish: the forgotten victims on our plate. The Guardian, September 14. Available at: https://www.theguardian.com/commentisfree/cif-green/2010/sep/14/fish-forgotten-victims. Accessed March 16, 2023.
Singer, P. 2011. Practical ethics. 3rd ed. Cambridge University Press.
Sloman, K. A., I. A. Bouyoucos, E. J. Brooks, and L. U. Sneddon LU. 2019. Ethical considerations in fish research. Journal of Fish Biology 94:556–577. doi:10.1111/jfb.13946.
Smith, J. L. B. 1968. Our fishes. Voortrekkerpers, Johannesburg.
Sneddon, L. U. 2002. Anatomical and electrophysiological analysis of the trigeminal nerve in a teleost fish, Oncorhynchus mykiss. Neuroscience Letters 319:167–171.
Sneddon, L. U. 2011. Cognition and welfare. Pages 405–434 in C. Brown, J. Krause, and K. Laland, editors, Fish cognition and behavior. 2nd ed., Wiley-Blackwell, Oxford.
Sneddon, L.U. 2011. Pain perception in fish: evidence and implications for the use of fish. Journal of Consciousness Studies 18(9-10):209–229.
Sneddon, L. U. 2019. Evolution of nociception and pain: evidence from fish models. Transactions of the Royal Society B 374:20190290. https://doi.org/10.1098/rstb.2019.0290.
Sneddon, L. U., V. A. Braithwaite, and M. J. Gentle. 2003a. Do fishes have nociceptors? Evidence for the evolution of a vertebrate sensory system. Proceedings of the Royal Society of London B 270:1115–1121. doi:10.1098/rspb.2003.2349.
Sneddon, L. U., V. A. Braithwaite, and M. J. Gentle. 2003b. Novel object test: examining pain and fear in the Rainbow Trout. Journal of Pain 4:431–440.
Sneddon, L. U., and C. Brown. 2020. Mental capacities of fishes. Pages 53–71 in L. S. M. Johnson et al., editors. Neuroethics and nonhuman animals: advances in neuroethics. Springer, Cham, NY.
Sneddon, L. U., R. W. Elwood, S. Adamo, and M. C. Leach. 2014. Defining and assessing animal pain. Animal Behaviour 97:201–212.
Sneddon, L. U., J. Lopez-Luna, D. C. Wolfenden, M. C. Leach, A. M. Valentim, P. J. Steenbergen, N. Bardine, A. D. Currie, D. M. Broom, and C. Brown. 2018b. Fish sentience denial: muddying the waters. Animal Sentience 21(1):1–11.
Sneddon, L. U., and D. C. C. Wolfenden. 2019. Ornamental fish (Actinopterygii). Pages 440–466 in J. Yeates, editor, Companion animal care and welfare: the UFAW companion animal handbook. John Wiley & Sons, Somerset, NJ.
Sneddon, L. U., D. C. C. Wolfenden, M. C. Leach, A. M. Valentim, P. J. Steenbergen, N. Bardine, D. M. Broom, and C. Brown. 2018. Ample evidence for fish sentience and pain. Animal Sentience 21(17). DOI: 10.51291/2377-7478.1375.
Sneddon, L. U., D. C. C. Wolfenden, and J. S. Thomson. 2016. Stress management and welfare. Fish Physiology 35:463–539.
Snow, P. J., M. B. Plenderleith, and L. L. Wright. 1993. Quantitative study of primary sensory neurone populations in three species of elasmobranch fish. Journal of Comparative Neurology 334:97–103.
Sørensen, C., J. B. Johansen, and Ø. Øverli. 2013. Neural plasticity and stress coping in teleost fishes. General and Comparative Endocrinology 181:25–34.
Theodoridi, A., A. Tsalafouta, and M. Pavlidis. 2017. Acute exposure to fluoxetine alters aggressive behavior in zebrafish and expression of genes involved in serotonergic system regulation. Frontiers in Neuroscience 11:223. https://doi.org/10.3389/fnins.2017.00223.
Thompson M., S. Van Wassenbergh, S. M. Rogers, S. G. Seamone, and T. E. Higham. 2018. Angling-induced injuries have a negative impact on suction feeding performance and hydrodynamics in Marine Shiner Perch, Cymatogaster aggregata. Journal of Experimental Biology 221:jeb180935. doi:10.1242/jeb.180935.
Veit, W., and B. Huebner. 2020. Drawing the boundaries of animal sentience. Animal Sentience 29(13): 342.
Vettese, T., B. Franks, and J. Jacquet. 2020. The great fish pain debate. Issues in Science and Technology (Summer):49–53.
Vonk, J. 2020. A fish eye view of the mirror test. Learning & Behavior 48:193–194.
Walster, C., E. Rasidi, N. Saint-Erne, and R. Loh. 2015. The welfare of ornamental fish in the home aquarium. Companion Animal 20:302–306.
Whitear, M. 1971. The free nerve endings in fish epidermis. Journal of Zoology London 163:231–236.
Woodruff, M. L. 2017. Consciousness in teleosts: there is something it feels like to be a fish. Animal Sentience 13(1). DOI: 10.51291/2377-7478.1198.
Harmful, poisonous, or very unpleasant
Establishing, relating to, or deriving from a standard or norm, especially of behavior
A sensory receptor for painful stimuli
Make or become accustomed or used to something
Similar in position, structure, and evolutionary origin but not necessarily in function
To suggest or assume as true as the basis for reasoning, discussion, or belief
Mistaken belief, especially one based on unsound argument
The ability to infer social relationships between individuals
Deriving pleasure from inflicting pain, suffering, or humiliation on others
Harvested in a traditional or non-mechanized way
Action of draining a person, animal, or organ of blood

