11 Integrating Fishers in the Management of Arapaima
Learning Objectives
- Investigate the significance of Arapaima fishing in the Amazon.
- Examine the role of the Arapaima, one of largest freshwater fish of the world, as an example of a flagship species.
- Appreciate the cultural significance of the Arapaima.
- Explain how we detect overfishing.
- Explain the benefits and successful application of principles for sustainable governance of common property resources.
- Explore gender differences in Arapaima fisheries.
11.1 People and Fish of Amazonia
The settlement of the Amazon region is a story of many people and their relationship with the rain forest and its resources. Fish were a dominant part of the diets of indigenous people. The Amazon River basin, also known as Amazonia, is one of the world’s largest river systems, with approximately 12 times the volume of water carried by the Mississippi River. At its mouth, one cannot see across the Amazon from one bank to the other. The Amazonia region, which includes the Amazon, Orinoco, and rivers of Guyana, has the richest freshwater fish fauna in the world! Amazonia is a cradle of biodiversity, with over 3,000 fish species and likely many more yet to be discovered. For example, more than 100 new fish species were described between 2017 and 2019 (Jézéquel et al. 2020). The fish fauna in parts of the Amazon basin is still in a relatively good state of conservation (Reis et al. 2016), and fish provide for many ecosystem services, such as nutrient cycling, grazing, seed dispersal, and essential nutrition and livelihoods for many people of Amazonia. Sustainable fisheries are essential for the food security of people of this region, and unsustainable land and water use practices threaten this important hot spot for fish conservation (Pelicice et al. 2017).
The first humans to migrate across the land bridge from Siberia to Alaska during the Pleistocene (at least 16,500 years ago) settled in western North America. By the late Pleistocene and early Holocene (~12,000 years ago), humans had migrated from North and Central America to South America, likely via the Isthmus of Panama (Hester 1966). Early humans likely domesticated manioc, maize, squash, and beans in addition to hunting, fishing, and gathering (Lombardo et al. 2020). By the time that explorers from Portugal discovered present-day Brazil in 1500, there were hundreds of native tribes inhabiting the region. Some experts speculate that there may have been 15 million Amerindians in the basin before Europeans arrived (Smith 1999). Fish were important wild food, as revealed by bones of many fish species, including small characiforms, catfish, and Arapaima, at archeological sites from 11,200 to 8,000 years ago (Roosevelt 1999).
Portuguese colonists bartered with the native peoples and developed a profitable export trade for brazilwood and other commodities. However, tensions soon developed, and the Portuguese colonists turned to violent confrontations with indigenous tribes. The custom of native peoples of frequently moving villages to prevent damage to local flora and fauna conflicted with the European system of private ownership and permanent settlements. Indigenous people explored the many rivers and developed villages, passing on specialized local knowledge of fishing and other essential products from the rivers, lakes, and forests. The Native Amazonian people love and live successfully in rain forest communities. However, violence and exposure to novel diseases, such as smallpox, led to gradual replacement of indigenous people with colonists from Europe and Africa in the 17th century. Land surrounding large human settlements became highly modified due to logging, livestock grazing, and commercial agriculture.
Indigenous peoples of the Amazonian floodplains are themselves a diverse group, called ribeirinho, or river settlers. Ribeirinhos live alongside the Amazonian floodplains and have intimate knowledge about the river and forest resources upon which their livelihoods depend (Moran 1993). Indigenous people settled in the flooded forest ecosystem, where they continue to live today with little advanced technology and live largely on cassava manioc (to derive flour, tapioca, and bread), wild fish, bush meat, and pequi fruit (Dufour et al. 2016; Schor and Azenha 2017). All indigenous groups recognize many wild plants and animals, their relations to soil quality, and their useful properties. Increasingly via cash trading, they also purchase canned goods, frozen chicken, dairy, and other refrigerated foods.
Today, the people of Amazonia include indigenous peoples and colonists, each with differing cultures. Modern Brazilians descend from Portuguese colonists, African enslaved people, intermarriage of both races with indigenous peoples, and recent immigration by other Europeans and Asians (Hemming 2020). Colonists cleared the floodplains for farming and engaged in slash-and-burn tactics for profit-driven cattle grazing or soy plantations; hence it is difficult for each group to understand the other. Historically, the indigenous people have suffered genocide, violence, and exploitation of their lands for mining, cattle ranching, logging, hunting, and big agriculture.
Brazil was ruled by a Portuguese monarchy for more than three hundred years before becoming independent in 1822. Millions of enslaved people were imported to work on coffee plantations, until slavery was outlawed in 1888. When Brazil began democratic rule in 1985, groups fought to get rights for indigenous people. Brazil’s constitution (1988) (1) declared that indigenous people were descendants of original Brazilians and hence owned lands, and (2) guaranteed respect for their way of life and provided exclusive use of the goods and resources on indigenous lands. Today every forest tribe has its land protected (Hemming 2020).
Approximately 89% of the population of Brazil now resides in urban areas, and strong rural-to-urban migration continues. Children of rural migrants are exposed to different food options in urban areas, leading to reduced fish consumption. Fishing pressure is focused on few species (Bayley and Petrere 1989), and overfishing is driven by the demand for fish from urban settlements (Tregidgo et al. 2017). Agriculture production has grown dramatically in Brazil, resulting in clear-cutting of mature forest to plant grains and raise beef cattle (Nepstad et al. 2014). Deforestation is only one of many threats to the Amazon region. Other threats to fisheries include overfishing, nonnative species, aquaculture, pollution, water diversions, habitat loss, mining, and poor management.

Fisheries, river and lake ecosystems, and wetlands of Amazonia support many regional economies and livelihoods of traditional and indigenous communities (Goulding 1996). Most fishing in rural communities is for subsistence to feed families, and only the surplus is sold. Fish are still the cheapest and most important source of animal protein in the central Amazon. Per capita fish consumption is high in the Brazilian Amazon, at 5.8 times the world average for riverine dwellers and 2.5 times the world average for urban dwellers (Isaac and Almeida 2011).
Subsistence fisheries are a large economic activity and livelihood component of rural communities. Globally, small-scale fisheries contribute to food security and employ 32 million fishers (World Bank 2012). However, fisheries management agencies collect incomplete statistics because small-scale fisheries tend to be physically remote and agencies lack sufficient human and financial resources (Berkes et al. 2001). Arapaima fishing illustrates how innovative approaches to fisheries governance may lead to recovery of overfished populations and alleviate poverty without major government intervention.
11.2 Arapaima: An Example Freshwater Megafauna and Flagship Symbol
Arapaima (pronounced “air-ah-pie-ma”) is one of the most acclaimed fishery resources of the Amazon region and has considerable socioeconomic importance. In Brazil and Colombia, they are called Pirarucu, a Portuguese name meaning red fish. Arapaima are called Paiche in Peru, Ecuador, Venezuela, and Bolivia, and sometimes simply Giant Arapaima. Its large size (up to 3+ meters and >200 kg) and the high quality of its flesh make it one of the most historically important and overexploited fisheries in South America. The people of Guyana call Arapaima Oma, or the “mother of all fish,” which serves as a local taboo against harvest.

Arapaima is an important flagship genus for flooded forest ecosystem and human floodplain communities. Flagship taxa are used as a symbol to promote conservation awareness (Caro 2010). Their large size makes them a true freshwater megafauna like crocodiles, river dolphins, and other large fish. Freshwater megafauna face many threats, and 71% of these species are in decline (He et al. 2017, 2018). Arapaima continue to face intense fishing throughout their range (Watson et al. 2021). However, freshwater megafauna like the Arapaima have fewer conservation resources and efforts than marine or terrestrial megafaunas.
Fishing, in general, and fishing for Arapaima in particular, is a central element of the local economy and culture in Amazonia. Because these fish are obligate breathers, they are traditionally harvested by fishers using harpoons at the time when they surface to breathe. Men typically fish from canoes and search for signs of Arapaima near the surface. As they near the Arapaima, the harpooner throws the harpoon by hand. This is a specialized type of fishing, and the local fishers possess knowledge of the behavior that increases their likelihood of catching one. With appropriate training, fishers’ participation in management processes can contribute to the conservation and governance of these small-scale fisheries.
Many populations of Arapaima have been driven to local extinction due to overfishing (Castello et al. 2015a; Gurdak 2019a; Watson et al. 2021; Freitas and Sousa 2021). Much of the catch is illegal, with most specimens being caught below the minimum size limit or during the closed season (Cavole et al. 2015). The small-scale fishers are geographically dispersed, and governments in these regions have insufficient resources to devote to enforcing fishing rules. The riverine fishers who target Arapaima are marginalized and have limited formal education. Yet, compliance with regulations is essential to prevent overfishing and local extinction.
Arapaima represent only a small fraction of the fisheries harvest, but they are culturally important and symbolic as a flagship genus of tropical South American fisheries and floodplain management and conservation. Reducing the threats to Arapaima will also provide protections for many of the highly migratory fish of the Amazon basin. Collectively, the migratory fish contribute most of the fishery’s landings in the basin (Duponchelle et al. 2021). Migratory fish depend on multiple, distant, but interconnected habitats during their life cycle. Any threat to one of the habitats or the corridor that connects them can influence these important food fish (Goulding et al. 2019).
11.3 Habits, Habitat, and Life History of Arapaima
Arapaima live in floodplain lakes that experience seasonal variation in water levels, ranging from 4 to 15 meters. The floodplain along the sediment-rich waters of the Amazon basin consists of seasonally inundated rain forests, lakes, and winding channels. The seasonal flood pulse creates a new and expanding littoral zone that moves with the rising waters. Seasonal flood pulses provide new nutrient, detritus, and sediment inputs from the main river channel and drive the high productivity of numerous prey fish of the Arapaima (Watson et al. 2013; Castello et al. 2015b; Carvalho et al. 2018). Arapaima are more abundant in deeper and larger lakes with more space and food (Arantes et al., 2013; Campos-Silva and Peres, 2016). Juvenile Arapaima in particular benefit from lakes with large littoral zones that move with rising water (Castello et al. 2019).
Large Arapaima are the ultimate ambush predators. They belong to a group of primitive bony fish known as bonytongue fish, because their tongues are used to crush prey against the roofs of their mouths. Smaller Arapaima are generalist feeders, consuming a variety of invertebrates, such as the Amazon River prawns, mayflies, and crickets, while larger Arapaima can consume larger prey, often catfish, cichlids, hatchetfish, and pacu (Watson et al. 2013; Carvalho et al. 2018; Jacobi et al. 2020). During low water periods, many isolated lakes can become hypoxic (i.e., low in oxygen). The air-breathing habit permits Arapaima to survive in such environments and prey on fish stressed by low oxygen.
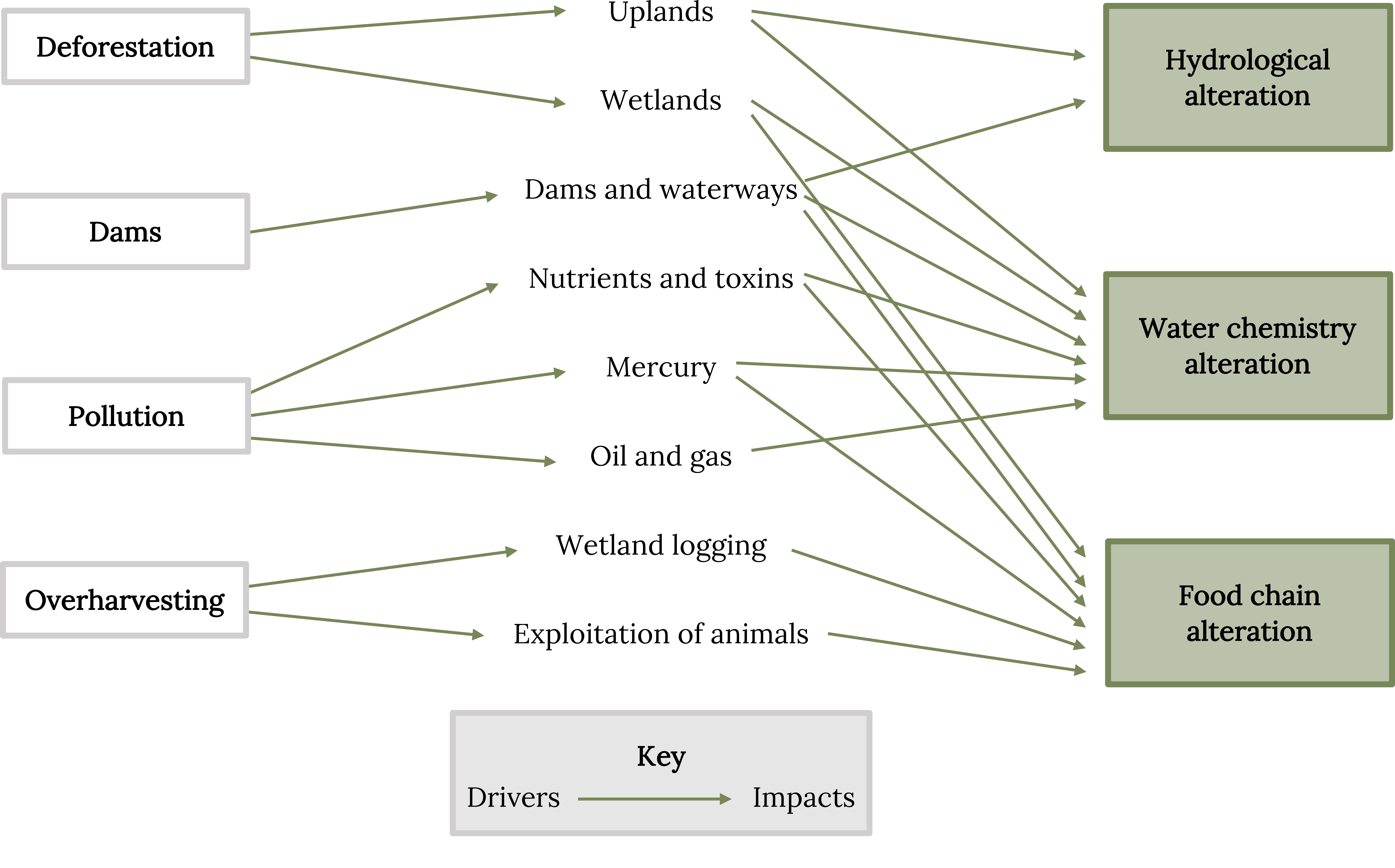
In addition to overfishing of Arapaima, many of its essential habitats are modified by deforestation, dams, pollution, and logging in nearby wetlands (Figure 11.3; Castello et al. 2013b; Castello and Macedo 2016; Pelicice et al 2017; Gurdak et al. 2019a). Because freshwater ecosystems are highly sensitive to human activities on water and on land, these growing impacts are currently a major constraint to conservation (Pelicice and Castello 2021). Human influences cause a complex chain of effects that alter the hydrology, water chemistry, and food webs of Amazon floodplain rivers. Current government policies, guided by short-term economic profits, ignore the scientific evidence of environmental degradation and threaten efforts to conserve and protect aquatic ecosystems and the many fishes that depend on them.

The life cycle of Arapaima is synchronized with the seasonal flooding cycle and consists of four main stages: (1) nest-bound embryo and sac fry, (2) adult-protected, schooling juveniles, (3) independent juveniles, and (4) reproductive adults (Figure 11.4). Although the Arapaima are among the largest freshwater fish in the world, their lifespan is only about 20 years. They attain reproductive maturity when they approach 150 cm in total length (TL) or age three or four. Size at reproductive maturity varies between 139 cm in the lower Amazon to 207 cm in the upper Amazon in Peru (Gurdak et al. 2019b). Arapaima migrate at the start of the rainy season in response to rising water levels and build nests in shallow, soft, sandy or muddy areas, usually under woody vegetation. Clearing a nest site likely serves to limit small predatory fish from eating eggs and larvae. Eggs are deposited in the nest by the female and fertilized by the male, and developing embryos are guarded by both parents. For such a large fish, fecundity is relatively low, with about 10,000 to 20,000 mature oocytes for an 80 kg female. However, the eggs are large (~2.5–3 mm) and hatch in about seven days to become sac fry.
The small fry are a dark color and stay near the parental male Arapaima’s head. The male’s head turns dark to help hide the fry. Males release a pheromone that attracts his offspring and keeps them close by as he guides his offspring into zooplankton-rich areas for feeding. The substance is referred to by local people as “Arapaima milk,” which may provide nutrition to young Arapaima as well as provide a means of chemical communications (Torati et al. 2017). Both parents continue to guard the juveniles as they school in search of food, but the female normally leaves after about one month, while the male stays with his offspring for up to three months. Juveniles are often preyed upon by other species of fish, particularly the abundant cichlid fish, such as the piranhas and Peacock Bass (Cichla or tucunaré).
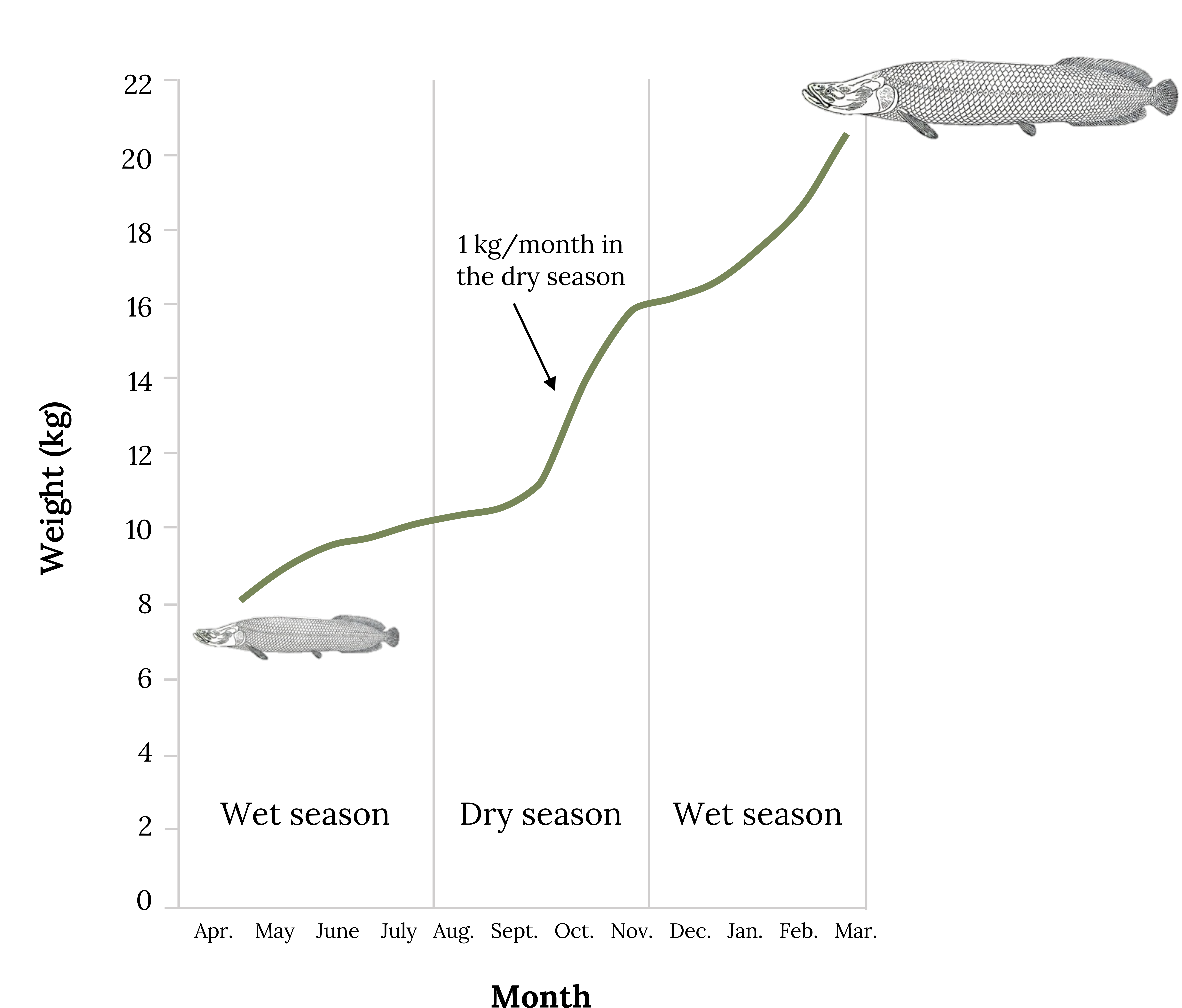
Growth is fast, and juveniles disperse and live independent of parents at about 50 cm total length. In the central Amazon, Arapaima may grow to 30 cm TL in 3 months and 88 cm and 20kg in a year (Figure 11.5; Arantes et al. 2010), which may be the fastest juvenile growth of any fish (Schwenke and Buckel 2008; Sakaris et al. 2019). Growth is faster in the dry season, as more prey are concentrated. As adults, Arapaima have few natural predators because of their tough layer of scales. Only the rain forest caiman is known to prey on adults. Arapaima scales are among the toughest biological materials in nature, and they protect them from the abundant piranha (Sherman et al. 2017; Yang et al. 2019).
11.4 Biogeography and Conservation Status of Arapaima
Arapaima gigas is the most well researched species of Arapaima. Ichthyologist Albert Günther (1868) declared with no rationale that it was the only valid species, a view that persisted for over 100 years only because scientists never questioned his claim. However, as many as three other species of Arapaima have been recently named in Brazil, Peru, and Guyana (Stewart 2013a, 2013b). The most recent was Arapaima leptosoma, found in the Solimões River in Brazil. Arapaima mapae comes from the Lago do Amapá in Brazil, from which it takes its scientific name. Arapaima agassizii was named after famous biologist Louis Agassiz. Although these four Arapaima species were described in the 1800s, it was their different characteristics that have allowed Donald J. Stewart to classify them separately in his recent work. Although there is still no consensus on Arapaima taxonomy (Farias et al. 2019), ongoing studies indicate that there may be up to six valid species. Donald J. Stewart admonishes other scientists to “Beware of conventional wisdom—what we know might be completely wrong” (Stewart 2013a).
Available evidence indicates that Arapaima populations are likely decreasing in the entire Amazon basin (Castello and Stewart 2010). Where data do exist, there is a preponderance of juveniles, indicative of overfishing. Current distributions of the species cannot be accurately mapped due to uncertainty on the taxonomy and geographical distribution. However, the findings to date highlight the urgent need for caution in translocations of individuals. Conservation status is determined based on levels of reduction in population size, geographic range occupied, and number of populations. The Brazilian Environment Institute (IBAMA) classified Arapaima gigas as an overfished species or a species threatened with overfishing (Nogueira et al. 2020). Arapaima gigas was listed on the CITES Appendix II since 1975. Species listed in CITES Appendix II may be exported only after a nondetriment finding is affirmed. However, data are deficient for status assessment on most wild Arapaima populations. Populations from Guyana were classified as “near threatened” (Watson et al. (2021). Different species and different populations of Arapaima exhibit key life-history and ecological differences that may be relevant to their conservation (Watson et al. 2016; Watson and Stewart 2020). Two other species, Arapaima agassizii and Arapaima leptosoma, are recognized by the Brazilian Red List as “Data Deficient.” Given the taxonomic uncertainties, fisheries management in this region currently refers to Arapaima only at the genus level (Arantes et al. 2021).
11.5 Vulnerability to Overfishing
Several characteristics of Arapaima make them highly vulnerable to overfishing. First, they are obligate air breathers and typically must surface every 5–15 minutes to gulp air; this makes them easy to locate. Second, the high-quality, boneless flesh is highly sought, making Arapaima a popular commercial food fish. Third, their skin can be used in the manufacturing of shoes, bags and clothing, and its scales and tongue are used in the manufacturing of nail files and ornaments. One hundred years ago, Arapaima dominated fisheries in the Amazon. Unregulated fishing with gill nets led to overfishing and many local extinctions, leading to a ban on fishing for Arapaima in 1986. Yet, gill nets are still used in the Amazon to capture other small fish and inadvertently capture juvenile Arapaima.
Characteristics of Arapaima that make them vulnerable to overfishing:
- Obligate air breathers
- High-quality flesh
- Skin and bones used in products
To protect rich native biodiversity in the Amazon, biological reserves were established. The largest is the Mamirauá Ecological Reserve, one-third of which is in flooded forest. This reserve is approximately half the area of New Jersey. The thrust of this reserve is the integration of local people into reserve management. In biological reserves, agreements guarantee indigenous fishers the exclusive right to fish (or to hunt) Arapaima but only with harpoons. Harpoons provide an efficient catch method for targeting Arapaima. The harpooners, referred to as laguistas, are specialists in handling harpoons and are familiar with the habits of the Arapaima (Sautchuk 2012). Searching for Arapaima involves catching the fish unaware, to facilitate approach and, ultimately, harpooning. Experienced fishers have an extraordinary ability to detect very subtle visual and acoustic information from surfacing Arapaima (Castello 2004).
Where management is weak or nonexistent and multiple fishers compete to catch fish, the large individuals are rapidly removed from the population and catch rates are barely sufficient to cover the costs of fishing, and fishers seek other areas. Remoteness of the fishing communities means that government presence and enforcement of regulations are lacking. Monitoring landings is practically impossible because of the decentralized and illegal nature of the trade. One survey from 81 fishing communities indicated that the local Arapaima stocks are depleted in 76% of the communities and overfished in 17% (Castello et al. 2015a). Only 5% were well managed and only 2% were unfished. Illegal fishing is still the principal threat to Arapaima populations (Castello and Stewart 2010; Cavole et al. 2015; Faria et al. 2018).
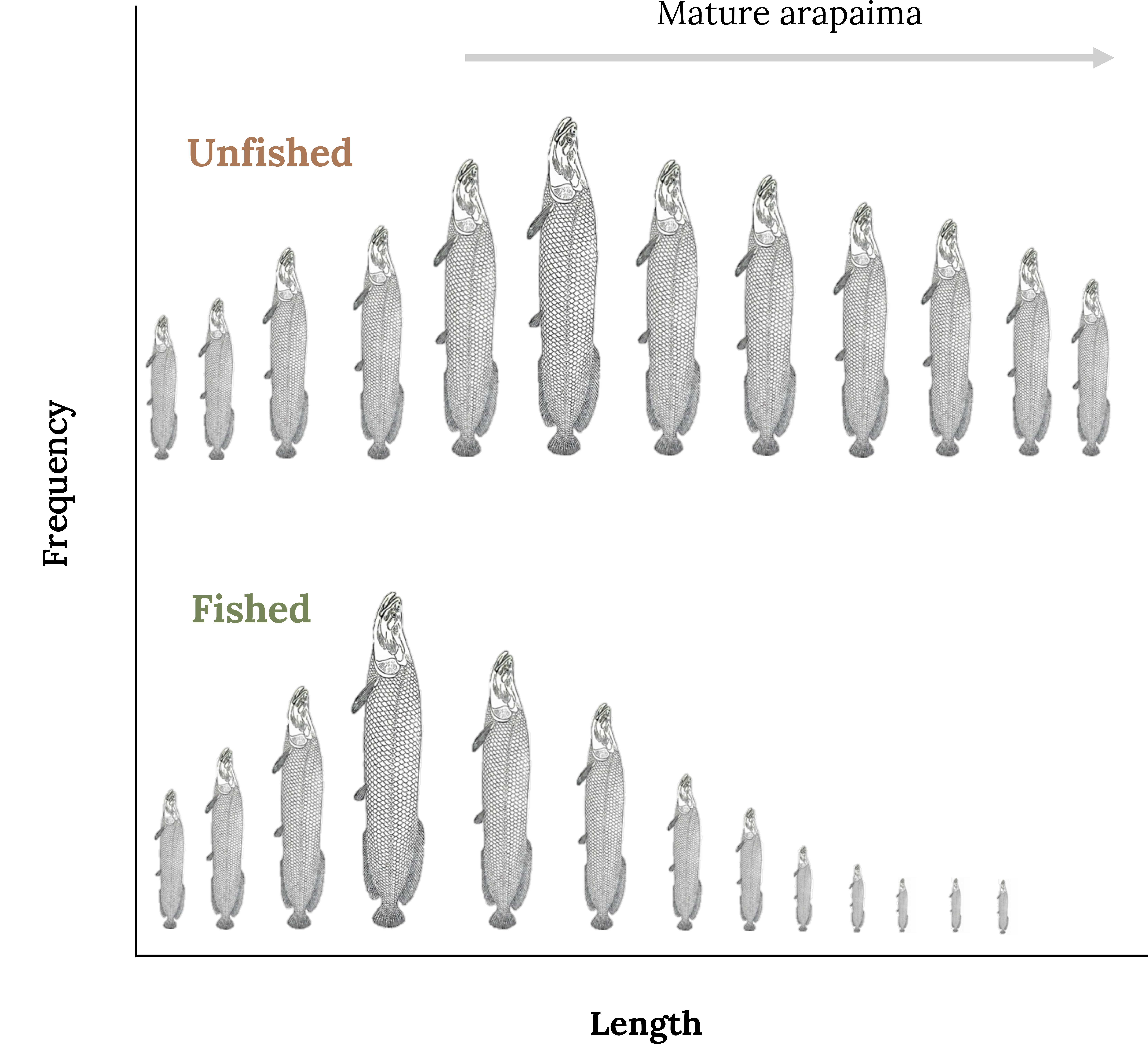
In many areas, Arapaima are poached before they are able to mature and spawn; in some populations, 80–90% are killed long before they mature (Figure 11.6). A fishery that includes many small, immature fish in the catch is subject to growth overfishing, where the fish are removed well before they reach sexual maturity and their full growth potential. The result is size and age truncation, which is prevalent and often severe in exploited fish populations (Barnett et al. 2017).
Old fish have disproportionate effects on population growth, and scientists are beginning to recognize the benefits of big, old, fat, fertile, female fish in the population (BOFFFF; Hixon et al. 2014). Removal of too many immature fish reduces the number of BOFFFFs so that replenishment potential is restricted. Larger Arapaima are likely more effective at producing more offspring and protecting young from many predators.
Questions to ponder:
What characteristics of the Arapaima make them particularly vulnerable to overfishing? How might you develop a monitoring program to determine if overfishing is occurring for Arapaima?

The principle regulatory measures for Arapaima have been closed fishing season during the high-water spawning seasons (December 1 to May 31) and a 1.5-meter minimum length limit. Arapaima gigas is coveted in the leather fashion industry for their unique skin pattern, and the leather trade has increased in recent decades (Figure 11.7). CITES (Convention on International Trade in Endangered Species of Wild Fauna and Flora) is an international agreement between governments to ensure that international trade in specimens of wild animals and plants does not threaten their survival. In the United States, legal and illegal trade of Arapaima is monitored using Law Enforcement Management Information System (LEMIS) data from the U.S. Fish and Wildlife Service (USFWS). Legal harvest of Arapaima must be conducted with a specific management plan, and their international commercialization is also under control. In Brazil, Arapaima leather yields higher prices per unit on international markets than Arapaima meat, and the leather products are more likely to get exported. Arapaima leather trade has increased in recent decades as a substitute for decline in leather from pangolin, the most heavily trafficked wild mammal (Heinrich et al. 2019).
Exports of Arapaima are only allowed if they are either wild caught from management areas or captive bred (Sinovas et al. 2017). However, a recent study from Brazil revealed that almost 80% of Arapaima landings were illegal (Cavole et al. 2015), which was observed to be the highest level of illegal fishing activity reported in the literature. Trade in Arapaima is still new and changing, and future trends and effects on populations need further study.
11.6 Incorporating Fishers in the Management of Arapaima Fishing in the Amazon
Small-scale fisheries are often poorly managed, yet they employ most of the world’s 51 million fishers, produce about half of the global reported catch, and provide food, income, and livelihood to about 1 billion people. Hardin’s “Tragedy of the Commons” argued that without government regulations or private ownership, overharvest of common property resources was inevitable. Federal governments own the fishery resources of the Amazon and, therefore, are responsible for setting rules that govern fish harvest. In Brazil, resource management is done by the Brazilian Institute of the Environment. Policies of this agency are based on a scientific management model in which government technocrats and field agents design, implement, and enforce fisheries management regulations. Consequently, fishing agreements made by local communities were initially thought to have no legal validity because communities had no right to regulate local fisheries (McGrath et al. 2015).
“Common property” refers to the right to use something in common with others. However, after collapse of major fisheries, some planners began to lose faith in government regulation of fishing. As an alternative, Elinor Ostrom in Governing the Commons (1990) advocated for community-based management (or comanagement) approaches to manage the common property resources. Community-based management has the potential to overcome the tragedy of the commons. One flaw in the tragedy of the commons idea is that it ignores the social relations that characterize fishers throughout the world. Fishers are subject to social pressures that shape their behavior. Therefore, efforts to reduce illegal fishing should focus on establishing and enforcing sanctions for fishers who violate rules and regulations.
Top-down policies, often referred to as Decide-Announce-Defend (DAD), as noted earlier, too often lead to abandoned or ineffective policies. The DAD method is not suited for fisheries where a wide range of technical, social, cultural, and economic factors are influencing the fishing status and alternatives. Implementation of regulations involves a lot of people, and most are not in an obvious command structure (Prince 2003; Walker 2009). The alternative participatory approach is Engage-Deliberate-Decide (EDD). Here the fishers choose whether to cooperate in a process to deliberate among alternative management interventions. Traditional knowledge held by the fishers may play important roles in creating alternative approaches. The approach is sometimes referred to as “two-eyed seeing” (Reid et al. 2021). An early proponent of two-eyed seeing, Dr. Albert Marshall, describes two-eyed seeing as “learning to see from one eye with the strengths of Indigenous knowledges and ways of knowing, and from the other eye with the strengths of mainstream knowledges and ways of knowing, and to use both these eyes together, for the benefit of all.” Whether or not comanagement regimes will prevent the tragedy of the commons depends on strong commitment from leadership to work with local stakeholders to develop and enforce quotas (Gutiérrez et al. 2011; McGrath et al. 2015; Campos-Silva et al. 2017).
Two-Eyed Seeing
“Two-eyed seeing” means that we will be learning to see from one eye with the strengths from indigenous knowledge and indigenous ways of knowing, while the other eye is using mainstream knowledge or Western ways of knowing. We use both of these ways of knowing (i.e., eyes) simultaneously. Hopefully, we’re learning more in this way. With indigenous people, the knowledge is all about transforming the holder of that knowledge. And then that holder will bear a responsibility to act on knowledge. It’s not a Western approach to knowledge where the knowledge is just put in a book for others to find. However, knowledge is there to be acted upon. Indigenous ways of knowing are more interconnected because it’s the people who are learning and sharing. It’s more holistic learning and occurs in many different ways. Indigenous knowledge is not hierarchically structured. For example, in Western scientific organizations, we have high-level scientists and low-level workers. In indigenous ways of knowing, everyone is fully engaged in the traditions and experience by which most indigenous people learn new things, whereas the Western ways of knowing are individualistic. We compartmentalize knowledge, especially as we develop scientific disciplines. Sociology, biology, physics, chemistry, and other sciences are part of the work in different laboratories. The science and technology disciplines are often male dominated, objective, and scientific. Ways of knowing in Western science are not necessarily better or worse, just different from the ways of knowing in indigenous societies.
Comanagement is an efficient management scheme across fishery types to avoid the tragedy of the commons. In comanagement, fishers collaborate with managers and scientists. Fishers enact their own management by self-regulating under the advice of scientists. Scientists and fishers work together in enacting the management. For many small-scale fisheries, comanagement can be the only way to manage fisheries where other more institutional form of controls are absent or ineffective. This is true in most tropical coastal and developing nations. To be successful, comanagement should (1) develop practices embedded locally, historically, and culturally; (2) focus on fishers; and (3) empower fishers in decision making (Castello et al. 2009). When expected benefits of managing a fishery exceed the perceived costs of investing in better rules and norms, most users and their leaders are likely to organize around a comanagement scheme. The following eight principles were developed to guide effective comanagement of Arapaima fishing (Ostrom 1990; Castello et al. 2009):
- Boundaries of resources and users are clearly defined.
- Rules are established to permit the resource to be exploited sustainably.
- Collective action is functional.
- Resources and behavior of fishers are monitored.
- Rule offenders are sanctioned.
- Conflict resolution mechanisms exist.
- Central governments authorize and recognize comanagement arrangements.
- Management tasks are organized and distributed at different institution levels.
In the case of managing harvests of the Arapaima, the native fishers of the community provide much of the management and enforcement, as government attempts to restrict fishing have been unsuccessful due to a lack of enforcement. From 1993 to 1995, only 30% of the harvested Arapaima were longer than the legal length limit (Castello et al. 2011). Efforts to engage the Arapaima fishers in an experimental management process began in the newly created Mamirauá Sustainable Development Reserve in 1998. Part of the reserve was zoned for sustainable use and allowed local people to harvest resources if rules were in place to assure sustainable harvest. The first challenge was to replace the view that native peoples of Amazonia were “backward” with an attitude of respect for their role in stewardship of the ecosystem. The second was to overcome the lack of data on Arapaima populations so that harvest quotas could be developed.
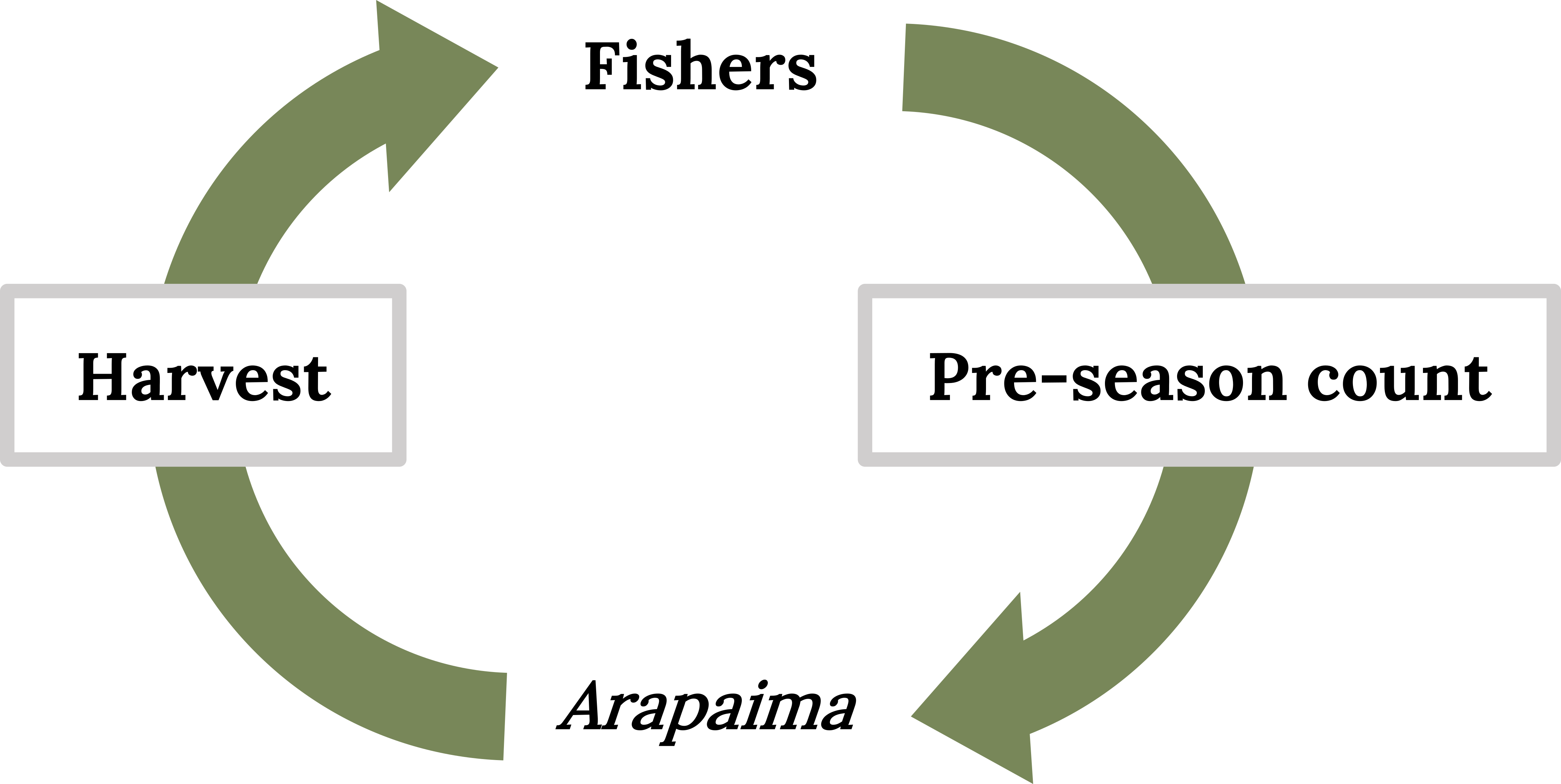
Comanagement began in four fishing communities in one area of the reserve. Here a counting method was developed so that experienced harpooners could accurately count surfacing Arapaima soon after dawn based on subtle visual and aural cues. The counts proved to be highly correlated with abundance estimates conducted by scientists (Castello 2004). Arapaima are relatively sedentary during the first hours after dawn, reducing the chance of those individuals being double counted (Campos-Silva et al. 2018). This counting method was ~200 times faster and less expensive than the marking and repeated capture method used by scientists. By counting numbers of Arapaima before the harvest season, fishers learned to self-manage populations. Briefly, annual counts made in lakes during the dry season were used to determine the harvest quotas for the next year (Figure 11.8). Mathematical analysis of Arapaima populations demonstrated that catch rates of about 25% of adults were likely to maximize the sustainable harvest (Castello et al. 2011). Government officials visited the fishers and the experimental management areas and were convinced that the management scheme was sound.
The results of this first experiment with comanagement of Arapaima fishing were indisputable (Figure 11.9). The total population of Arapaima increased 9-fold, and the harvest quotas increased 10-fold within seven years. In addition, the number of fishers participating in management more than doubled, and the per capita income of fishers increased 8-fold (Viana et al. 2004; Castello et al. 2009). Arapaima fishers were more engaged in this new management scheme, and there were fewer violations of the newly formulated rules.
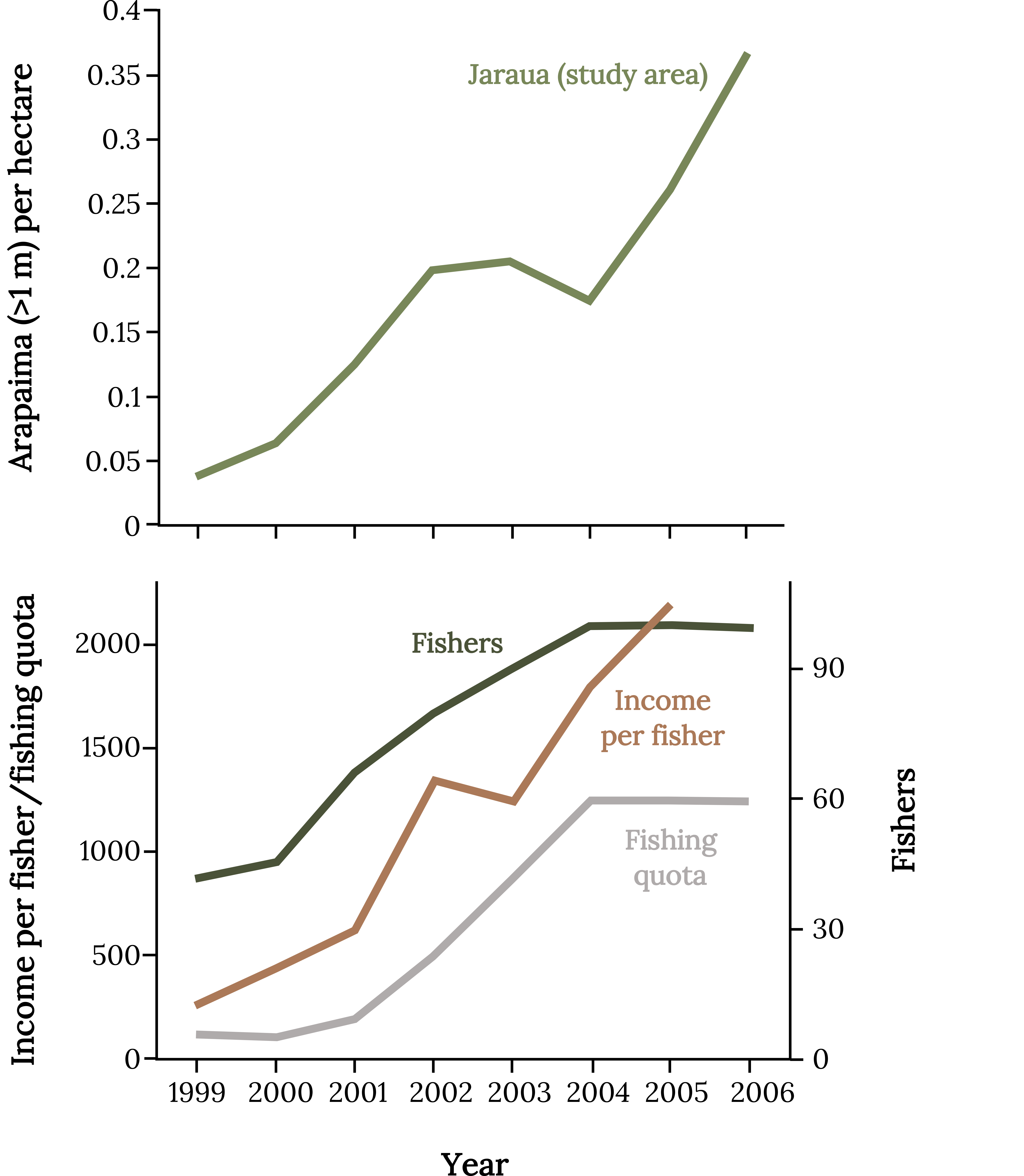
This type of comanagement scheme has proven successful in several different fishing communities that target Arapaima. For example, community-protected lakes in the western Brazilian Amazon had 33 times more Arapaima individuals than open-access lakes (Campos-Silva and Peres 2016). Similar responses to comanagement were observed elsewhere, leading to increases in the household income of Arapaima fishers (Oviedo et al. 2015, 2016; Petersen et al. 2016; Gurdak et al. 2019a; Watson et al. 2021; Gurdak et al. 2022). In addition, comanagement schemes resulted in time savings in fishing families, permitting more time for alternative pursuits, such as agriculture and cattle grazing (Schons et al. 2020). If the comanagement scheme could be implemented widely, the restored and well-managed Arapaima fisheries could yield as much as U.S. $30 million per year (Castello et al. 2011).
Experiences with comanagement of Arapaima demonstrated the importance of bridging knowledge across stakeholders, such as inviting government officials to observe the monitoring practices. Also, the scheme would be ineffective if not for the melding of the unique skills fishers can offer in conducting fish counts with scientific knowledge to estimate allowable catch levels (Castello et al. 2011b). Comanagement of Arapaima fishing can promote a more just distribution of benefits while recognizing cultural and gender differences among fishers (Lopes et al. 2021). Incorporating Ostrom’s design principles did increase the density of Arapaima, and future refinements should emphasize defining boundaries and formulating graduated sanctions for violators (Arantes et al. 2021).
Among the indigenous people, fishing and hunting are always done by males, whereas domestic tasks, such as getting water, gathering firewood, and childcare are principally performed by females (Meggers 1996). Processing of fish captured by males is a task done mostly by females. Because the most valuable catches are sold, Arapaima fishing provides one of the very few sources of income for females. After comanagement for Arapaima began, both men and women showed increased interest in participating in the local fishing association, and female income increased eightfold (Freitas et al. 2020).
Question to ponder:
What elements of comanagement of Arapaima do you think are most important for conservation?
11.7 Culture of Arapaima
Arapaima meat is in great demand because it is boneless, odorless, mild, low in fat content, and high in protein. Therefore, it demands a high price in the “gourmet” restaurant market. International market price is U.S. $20–25 per kilogram in Europe and the United States and $12–15 per kilogram in South American cities (FAO 2022). Arapaima are also pet traded in Europe, North America, South America, and Asia. Demand for juvenile Arapaima as ornamental fish is also high, partly because as obligate air breathers they tolerate hypoxic (low levels of dissolved oxygen) water uninhabitable by other fish (Ohs et al. 2021). As Arapaima were supplied to ornamental fish breeders in other countries, some escaped and established populations in Java and Sumatra in Indonesia (Marková et al. 2020). While threatened in its native range, the Arapaima populations elsewhere have spread rapidly and become invasive. This phenomenon is called the “biodiversity conservation paradox”—species at risk in their native range are abundant in other settings.
Aquaculture of Arapaima for food has been nonintensive and generally conducted in ponds or in net cages in reservoirs (dos Santos et al. 2014). The production cycle begins when breeding size fish are allowed to breed in ponds and offspring are stocked in tanks, net cages, or ponds and fed pellets. A major barrier to successful aquaculture of Arapaima is the production of a sufficient number of juveniles for stocking. Fry survival is high only in the early months of the flooding season (Núñez et al. 2011). Closed recirculating aquaculture systems show promise for increasing survival of fry and juveniles (Burton et al. 2016). Within 14 months after egg hatching, Arapaima attain a marketable size of 10–15 kg (22–33 pounds) and 110–120 cm (43–47 inches) (Núñez 2012; Ohs et al. 2021).
Brazil has invested in rapid expansion of new aquaculture facilities in public waters (Lima-Junior et al. 2018). However, poor aquaculture management practices and aquaculture of nonnatives and water use conflicts are troubling in this megadiverse region. Government support for aquaculture has decreased in the past decade (Nobile et al. 2020). Revised laws that foster aquaculture have encouraged farmers to raise tilapia and other nonnative fish without containment systems to prevent escapes (Padial et al. 2017).
Arapaima culture operations are still in the early phase of development, and many challenges remain. Aquaculture production in Brazil is based largely on nonnative species (Lima et al. 2018). As many as 501 nonnative species have been imported for the ornamental trade. Native species, such as Arapaima, have great, unrealized potential to contribute to food security and poverty reduction, if integrated with national and local development plans for biodiversity protection (Schaefer et al. 2012). Aquaculture production of Arapaima is still relatively low compared to the potential and represents about 6% of total farmed fish production in Brazil (Nobile et al. 2020). Exports of farmed Arapaima are sold in high-end supermarkets, such as Whole Foods Market®, which uses a “Responsibly Farmed” logo to promote farmed Arapaima.
Question to ponder:
What concerns would you have about culture of nonnative fish species in Brazil’s aquaculture industry?
11.8 Fly-Fishing Tourism Targeting Arapaima
Sportfishing for Arapaima is not a part of local indigenous culture. Yet, Arapaima are already providing benefits from ecotourism, such as fish watching and catch-and-release sportfishing in the Rewa and Rupununi rivers of Guyana. Approximately 89% of Arapaima caught by fly-fishing survived release (Lennox et al. 2018). Obligatory air-breathing habits of Arapaima mean that fishing guides must always hold fish at the surface, enabling fish to access air for three or four breaths (typically) prior to release. Adopting similar comanagement principles, some indigenous peoples have developed ecotourism lodges, which cater to foreign visitors and provide employment and income to indigenous communities. This approach promotes support for local culture and local ecosystems while permitting sustainable tourism. Use of traditional ecological knowledge to develop tourism based on nature and recreational fishing represents an innovative approach to economic development in rural parts of Amazonia. Here the local people serve as fishing guides, and fly-fishing and catch-and-release practices assure that released Arapaima will survive. This type of nature tourism is likely to increase in popularity and participation in Guyana, where the Arapaima is protected by national legislation but recreational fishing is still permitted. The hope of conservationists is that recreational fishing will encourage anglers to support Arapaima conservation via protection of habitats, river-floodplain connections, and avoiding illegal trade.
Profile in Fish Conservation: Leandro Castello, PhD

Leandro Castello, is Associate Professor of Fish Conservation at Virginia Tech. He is from Brazil, and from an early age he loved to be in or near the water and wanted to work in a field that explored the roles of fish and fishing in aquatic ecosystems. His early exposure to fish showed him there were other worlds to explore. When diving, he loved the feeling of being underwater, where fish and other aquatic life often move slowly and make many sounds in this foreign world.
Castello began to study Arapaima after the Mamirauá Sustainable Development Reserve was established. Here he developed a keen eye for seeing the big picture and making connections among different elements in the social and ecological parts of the region. In fisheries and fish conservation, too often people focus exclusively on the fish and ignore the connections between fish and people and the ecosystems that sustain them both.
Dr. Castello worked directly with local fishers to observe their fishing techniques and assist in training to determine if fishers could accurately count Arapaima before harvest season to derive a harvest quota. His collaborations with local Arapaima fishers led to the first evaluation of community-based management of Arapaima fishing. The initial findings from his and other evaluations of Arapaima comanagement have achieved remarkable social and ecological outcomes. As a result, poverty has been alleviated in many rural communities of Amazonia, as fishing benefits can now be sustained.
He has also studied the migration habits of Arapaima, which required many hours searching for tagged Arapaima. In doing so, he noticed the curiosity of Arapaima, as they would approach the canoe and watch what he and his field partners were doing. He also witnessed the learning capabilities in Arapaima. These fish prove to be very difficult to capture with seine nets. When corralled in a net, they either bury their bodies in bottom muds or jump over the seine net. Efforts to culture Arapaima have provided other opportunities to observe their habituation and social learning.
Translating scientific knowledge into workable policies and practices will serve to facilitate conservation in the face of the major environmental challenges of our times. Castello’s developing body of research and outreach addresses the gap between science and policy. This “science-policy gap” is often considered in negative terms, thereby increasing anxiety among early career scientists seeking to influence policy. Through persistence in efforts to develop trusted relationships needed for participatory management, he has pioneered joint learning and reflection among stakeholders. Along with many collaborators, Castello has successfully influenced policy and practices in Amazonia, and the effects of comanagement of Arapaima fishing provide others with a sense of optimism. His influential studies of tropical, small-scale fisheries provide many lessons to apply to conservation and restoration of exploited fisheries. The small-scale fisheries he studies are extremely neglected by most scientists, society, and governments. Yet, these complex systems are filled with mysteries yet to be fully understood.
Leandro Castello teaches courses in fisheries techniques and systems ecology in conservation. He works diligently to instill a sense of service in his students. Consequently, his students and colleagues are making a difference in promoting better approaches to conservation. In addition to managing to prevent overharvest, his research has demonstrated the need for increased protection of floodplain forests to benefit food, income, and livelihood of local fishing. These advances made by Castello and his colleagues come at a time when environmental policies in Brazil are unfriendly to environmental protection and push for more mining, hydropower development, deforestation, and agribusiness. His research and writings provide a framework for reversing unprecedented degradation of freshwater ecosystems in the Amazon basin.
Leandro recognizes the many connections between existing and proposed hydropower dams and ongoing land cover changes and climatic shifts. For example, with other world-renowned scientists, he provided advice for balancing the need for hydropower with biodiversity conservation in some of the largest and most threatened rivers of the world. He and his scientific colleagues called for mitigation of environmental impacts from human developments in the Amazon, Congo, and Mekong rivers. These three river basins hold roughly one-third of the world’s freshwater fish species, most of which are not found anywhere else. Consequently, the siting of future hydropower dams will be critically important for conserving biodiversity. Many other fish that feed and sustain us, such as less charismatic forage fish (often smelly and slimy), need more attention by scientists and conservationists. The strong role of hatcheries in fish conservation and management is a North American legacy. However, in most other parts of the world, fish provide subsistence and a means for a livelihood and poverty reduction.
Key Takeaways
- Arapaima comprise a prime example of a threatened freshwater megafauna (i.e., animals ≥ 30 kg) for which conservation status evaluations are needed.
- Arapaima are highly vulnerable to overfishing due to obligate air breathing, large size, and high-quality meat.
- Arapaima prefer large floodplain lakes with abundant macrophytes for spawning and juveniles.
- Obligate air breathing means that Arapaima must surface every 5 to 15 minutes to gulp air, thereby exposing themselves to specialist harpoon fishers.
- With appropriate training, fishers’ participation in management processes can contribute to the conservation of small-scale fisheries.
- Arapaima represent a culturally important, symbolic flagship genus that serves to support floodplain management and conservation.
- Illegal harvest and overfishing greatly reduce the economic returns from Arapaima, often leading to their local extirpation.
- Including local stakeholders in conservation planning of Amazonian floodplains leads to restoration of Arapaima populations while alleviating poverty among fisherfolk.
- Involvement of indigenous communities in management is a significant step (two-eyed seeing) toward sustainable fisheries that should continue to be promoted.
This chapter was reviewed by Leandro Castello.
Long Descriptions
Figure 11.3: 1) Deforestation impacts uplands and wetlands, which impacts water chemistry and food chain; 2) dams impact dams and waterways, which impact hydrological alteration; 3) pollution impacts nutrients and toxins, mercury, and oil and gas, which impacts water chemistry and food chain; 4) overharvesting impacts wetland logging and exploitation of animals, which impacts food chain alteration. Jump back to Figure 11.3.
Figure 11.4: Life cycle of arapaima divided into four main stages: 1) nest-bound embryo and sac fry (~7 days); 2) adult-protected, schooling juveniles up to age 6 months); 3) independent juveniles (up to age 3-4 years); 4) reproductive adult (aged 3-4 years or more). Jump back to Figure 11.4.
Figure 11.5: Growth of juvenile arapaima rises substantially during the dry season with a growth of 1 kg/month and heightened growth continues into the wet season. Jump back to Figure 11.5.
Figure 11.6: Two length frequency distributions contrasting fished and unfished arapaima populations. Length of fished arapaima reach their maximum frequency before unfished arapaima. Jump back to Figure 11.6.
Figure 11.9: Top graph: x-axis shows year, y-axis shows arapaima (>1 m) per hectare. Jaraua (study area) increases consistently, with a decrease from 2003-2004. Bottom graph: x-axis shows year, y-axis shows income per fisher/fishing quota. Fishers, fishing quota, and income per fisher all increase. Income per fisher decreases from 2002-2003 and passes fishers in 2005. Jump back to Figure 11.9.
Figure References
Figure 11.1: Indigenous and ribeirinho people travel on rivers of Brazil in a voadeira, a motorized canoe. Fernando C. C. Castro, 2020. CC BY-SA 4.0. https://commons.wikimedia.org/wiki/File:Parque_Nacional_de_Anavilhanas_Fernando_Carvalheiro_Coelho_Castro_(01).jpg.
Figure 11.2: Arapaima gigas displayed in the Siam Centre, Bangkok. Bjoertvedt, 2009. CC BY-SA 3.0. https://commons.wikimedia.org/wiki/File:Arapaima_gigas_01.JPG.
Figure 11.3: Schematic diagram of the main drivers influencing freshwater ecosystems in the Amazon. Kindred Grey, 2022. Adapted under fair use from The Vulnerability of Amazon Freshwater Ecosystems, by Castello et al., 2012. https://doi.org/10.1111/conl.12008.
Figure 11.4: The generalized life cycle of the Arapaima can be divided into four main stages: (1) nest-bound embryo and sac fry, (2) adult-protected, schooling juveniles, (3) independent juveniles, and (4) reproductive adults. Kindred Grey, 2022. CC BY 4.0. Adapted under fair use from Evidence of Recoveries from Tropical Floodplain Fisheries: Three Examples of Management Gains for South American Giant Arapaima, by Gurdak et. al., 2019. https://doi.org/10.47886/9781934874554.ch11. Includes Arapaima gigas, by Lankester Edwin Ray, 1908, public domain. https://commons.wikimedia.org/wiki/File:Arapaima_gigas1.jpg.
Figure 11.5: Juvenile Arapaima exhibit the fastest growth recorded in fish, reaching 15 kg or larger within the first year. Kindred Grey, 2022. CC BY 4.0. Data from Seasonality Influence on Biochemical and Hematological Indicators of Stress and Growth of Pirarucu (Arapaima gigas), an Amazonian Air-Breathing Fish, by Bezerra et. al., 2014. CC BY 3.0. https://doi.org/10.1155/2014/541278. Includes Arapaima gigas, by Lankester Edwin Ray, 1908, public domain. https://commons.wikimedia.org/wiki/File:Arapaima_gigas1.jpg.
Figure 11.6: Theoretical length frequency for unfished (top) and fished (bottom) populations of Arapaima. Kindred Grey, 2022. CC BY 4.0. Includes Arapaima gigas, by Lankester Edwin Ray, 1908, public domain. https://commons.wikimedia.org/wiki/File:Arapaima_gigas1.jpg.
Figure 11.7: Boots made from Arapaima leather, by Lucchese Boots, advertised on U.S. eBay website. Heinrich et. al., 2019. CC BY 4.0. https://doi.org/10.1111/csp2.75.
Figure 11.8: Integrating fishers who conduct counts of Arapaima prior to the fishing season in order to set harvest quotas. Kindred Grey, 2022. CC BY 4.0.
Figure 11.9: Responses of the Arapaima population, number of fishers, income per fisher, and fishing quota to the experimental comanagement process in the newly created Mamirauá Sustainable Development Reserve. Kindred Grey, 2022. CC BY 4.0. Data from Lessons from Integrating Fishers of Arapaima in Small-Scale Fisheries Management at the Mamiraua´ Reserve, Amazon, by Castello et. al., 2008. https://doi.org/10.1007/s00267-008-9220-5.
Figure 11.10: Leandro Castello, PhD. Used with permission from Leandro Castello. Photo by Jorge Pablo Castello. CC BY 4.0.
Text References
Arantes, C. C., L. Castello, X. Basurto, N. Angeli, A. Sene-Haper, and D. G. McGrath. 2021. Institutional effects on ecological outcomes of community-based management of fisheries in the Amazon. Ambio 51(3):678–690. DOI: 10.1007/s13280-021-01575-1.
Arantes, C. C., L. Castello, M. Cetra, and A. Schilling. 2013. Environmental influences on the distribution of Arapaima in Amazon floodplains. Environmental Biology of Fishes 96:1257–1267.
Arantes, C. C., L. Castello, D. J. Stewart, M. Cetra, and H. L. Queiroz. 2010. Population density, growth and reproduction of Arapaima in an Amazonian river-floodplain. Ecology of Freshwater Fish 19:455–465.
Araripe, J., P. S. do Rêgo, H. Queiroz, I. Sampaio, and H. Schneider. 2013. Dispersal capacity and genetic structure of Arapaima gigas on different geographic scales using microsatellite markers. PLoS ONE 8(1):e54470. https://doi.org/10.1371/journal.pone.0054470.
Barnett, L. A. K., T. A. Branch, R. A. Ranasinghe, and T. E. Essington. 2017. Old-growth fishes become scarce under fishing. Current Biology 27:2843–2848.
Bayley, P. B., and M. Petrere Jr. 1989. Amazon fisheries: assessment methods, current status and management options. Canadian Special Publication of Fisheries and Aquatic Sciences 1989: 385–398.
Berkes, F., R. Mahon, P. McConney, R. Pollnac, and R. Pomeroy, editors. 2001. Managing small-scale fisheries: alternative directions and methods. International Development Research Centre, Ottawa.
Burton, A. M., E. M. Calderero, R. E. B. Moran, R. L. A. Sánchez, U. T. A. Villamar, and N. G. O. Torres. 2016. A simple and low-cost recirculating aquaculture system for the production of Arapaima gigas juveniles. Revista Internacional de Investigación y Docencia 1(4):49–54.
Campos-Silva, J. V., J. E. Hawes, and C. A. Peres. 2018. Population recovery, seasonal site fidelity, and daily activity of piraucu (Arapaima spp.) in an Amazonian floodplain mosaic. Freshwater Biology 64:1255–1264.
Campos-Silva, J. V., and C. A. Peres. 2016. Community-based management induces rapid recovery of a high-value tropical freshwater fishery. Scientific Reports 6:34745. https://doi.org/10.1038/srep34745.
Campos-Silva, J. V., C. A. Peres, A. P. Antunes, J. Valsecchi, and J. Pezzuti. 2017. Community-based population recovery of overexploited Amazonian wildlife. Perspectives in Ecology and Conservation 15:266–270. https://doi.org/10.1016/j.pecon.2017.08.004.
Caro, T. 2010. Conservation by proxy: indicator, umbrella, keystone, flagship, and other surrogate species. Island Press, Washington, D.C.
Carvalho, F., M. Power, B. R. Forsberg, L. Castello, E. G. Martins, and C. E. C. Freitas. 2018. Trophic ecology of Arapaima sp. in a ria lake—river–floodplain transition zone of the Amazon. Ecology of Freshwater Fish 27:237–246. https://doi.org/10.1111/eff.12341.
Castello, L. 2008. Lateral migration of Arapaima gigas in floodplains of the Amazon. Ecology of Freshwater Fish 17:38–46. https://doi.org/10.1111/j.1600-0633.2007.00255.x.
Castello, L. 2004. A method to count pirarucu Arapaima gigas: Fishers, assessment, and management. North American Journal of Fisheries Management 24:379–389. https://doi.org/10.1577/m02-024.1.
Castello, L. 2021. Science for conserving Amazon freshwater ecosystems. Aquatic Conservation: Marine and Freshwater Ecosystems 31: 999–1004.
Castello, L., C. C. Arantes, D. G. McGrath, D. J. Stewart, and F. S. de Sousa. 2015. Understanding fishing-induced extinctions in the Amazon. Aquatic Conservation: Marine and Freshwater Ecosystems 25:587–598. https://doi.org/10.1002/aqc.2491.
Castello L., P. B. Bayley, N. N. Fabré, and V. S. Batista. 2019. Flooding effects on a long-lived, heavily exploited fish population of the central Amazon. Reviews in Fish Biology and Fisheries 29(6):1–14. https://doi.org/10.1007/s11160-019-09559-x.
Castello, L., V. J. Isaac, and R. Thapa. 2015. Flood pulse effects on multispecies fishery yields in the lower Amazon. Royal Society Open Science 2(11):150299. https://doi.org/10.1098/rsos.150299.
Castello, L., D. G. McGrath, C. C. Arantes, and O. T. Almeida. 2013a. Accounting for heterogeneity in small-scale fisheries management: the Amazon case. Marine Policy 38:557–565.
Castello, L., D. G. McGrath, L. L. Hess, M. T. Coe, P. A. Lefebvre, P. Petry, M. N. Macedo, V. F. Renó, and C. C. Arantes. 2013b. The vulnerability of Amazon freshwater ecosystems. Conservation Letters 6:217–229.
Castello, L., and D. J. Stewart. 2010. Assessing CITES non-detriment findings procedures for Arapaima in Brazil. Journal of Applied Ichthyology 26:49–56. https://doi.org/10.1111/j.1439-0426.2009.01355.x.
Castello, L., D. J. Stewart, and C. C. Arantes. 2011. Modeling population dynamics and conservation of Arapaima in the Amazon. Reviews in Fish Biology and Fisheries 21:623–640.
Castello, L., J. P. Viana, G. Watkins, M. Pinedo-Vasquez, and V. A. Luzadis. 2009. Lessons from integrating fishers of Arapaima in small-scale fisheries management at the Mamirauá Reserve, Amazon. Environmental Management 43:197–209.
Cavole, L. M., C. C. Arantes, and L. Castello. 2015. How illegal are tropical small-scale fisheries? An estimate for Arapaima in the Amazon. Fisheries Research 168:1–5. https://doi.org/10.1016/j.fishres.2015.03.012.
dos Santos, C. H. A., A. dos Santos, C. S. de Sá Leitão, M. N. Paula-Silva, and V. M. F. Almeida-Val 2014. Genetic relationships between captive and wild subpopulations of Arapaima gigas (Schinz, in Cuvier, 1822). International Journal of Fisheries and Aquaculture 6:108–123.
Dufour, D. L., B. A. Piperata, R. Murrieta, W. M. Wilson, and D. Williams. 2016. Amazonian foods and implications for human biology. Annals of Human Biology 43:330–348.
Duponchelle, F., V. J. Isaac, R. C. Doria, P. A. Van Damme, G. A. Herrera-R, E. P. Anderson, R. E. A. Cruz, M. Hauser, T. W. Hermann, E. Agudelo, C. Bonilla-Castillo, R. Barthem, C. E. C. Freitas, C. García-Dávila, A. García-Vasquez, J.-F. Renno, and L. Castello. 2021. Conservation of migratory fishes in the Amazon basin. Aquatic Conservation: Marine and Freshwater Ecosystems 31:1087–1105.
FAO. 2022. Arapaima gigas. Cultured Aquatic Species Information Programme. Text by J. Nuñez. Fisheries and Aquaculture Division, Rome. Available at: https://www.fao.org/fishery/en/culturedspecies/arapaima_gigas/en.
Farias, I. P., S. Willis, A. Leão, J. T. Verba, M. Crossa, F. Foresti, F. Porto-Foresti, I. Sampaio, and T. Hrbek. 2019. The largest fish in the world’s biggest river: genetic connectivity and conservation of Arapaima gigas in the Amazon and Araguaia-Tocantins drainages. PLoS ONE 14(8):e0220882. https://doi.org/10.1371/journal. pone.0220882.
Freitas, C. T., P. F. M. Lopes, J. V. Campos-Silva, M. M. Noble, R. Dyball, and C. A. Peres. 2020. Comanagement of culturally important species: a tool to promote biodiversity conservation and human well-being. People and Nature 2:61–81.
Freitas, H. C. P., and R. G. C. Sousa. 2021. Closed fishing season law a positive instrument to minimize illegal fishing of the remaining stock of Arapaima sp. in the Brazilian Amazon. Revista Ibero Americana de Ciências Ambientais 12:484–494. http://doi.org/10.6008/CBPC2179-6858.2021.001.0039.
Goulding, M., N. J. H. Smith, and D. J. Mahar. 1996. Floods of fortune: ecology and economy along the Amazon. Columbia University Press, New York.
Goulding, M., E. Venticinque, M. L. de B. Ribeiro, R. B. Barthem, R. G. Leite, B. Forsberg, P. Petry, U. L. da Silva-Júnior, P. S. Ferraz, and C. Cañas. 2019. Ecosystem-based management of Amazon fisheries and wetlands. Fish and Fisheries 20:138–158.
Gurdak, D. J., C. C. Arantes, L. Castello, D. J. Stewart, and L. C. Watson. 2019a. Evidence of recoveries from tropical floodplain fisheries: three examples of management gains for South American giant Arapaima. Pages 267–295 in C. C. Krueger, W. W. Taylor, and S-J. Youn, editors, From catastrophe to recovery: stories of fishery management success, American Fisheries Society, Bethesda, MD.
Gurdak, D. J., D. J. Stewart, L. Castello, and C. C. Arantes. 2019b. Diversity in reproductive traits of arapaima (Arapaima spp., Müller, 1843) in Amazonian várzea floodplains: conservation implications. Aquatic Conservation 29(2):245–257. https://doi.org/10.1002/aqc.3030.
Gurdak, D. J., D. J. Stewart, and M. Thomas. 2022. Local fisheries conservation and management works: implications of migrations and site fidelity of Arapaima in the lower Amazon. Environmental Biology of Fishes 105:2119–2132. https://doi.org/10.1007/s10641-021-01171-y.
Gutiérrez, N. L., R. Hilborn, and O. Defeo. 2011. Leadership, social capital and incentives promote successful fisheries. Nature 470:386–389.
He, F., C. Zarfl, V. Bremerich, A. Henshaw, W. Darwall, K. Tockner, and S. C. Jähnig. 2017. Disappearing giants: a review of threats to freshwater megafauna. Wires Water 4(3):e1208. https://doi.org/10.1002/wat2.1208.
He, F., V. Bremerich, C. Zarfl, J. Geldman, S. Langhans, J. N. W. David, W. Darwall, K. Tockner, and S. C. Jähnig. 2018. Freshwater megafauna diversity: patterns, status and threats. Diversity and Distributions 24:1395–1404.
Heinrich, S., J. V. Ross, and P. Cassey. 2019. Of cowboys, fish, and pangolins: US trade in exotic leather. Conservation Science and Practice 1:e75. https://doi.org/10.1111/csp2.75.
Hemming, J. 2020. People of the rainforest: The Villas Boas brothers, explorers and humanitarians of the Amazon. Oxford University Press.
Hester, J. T. 1966. Late Pleistocene environments and early man in South America. American Naturalist 100:377–388.
Hixon, M. A., D. W. Johnson, and S. M. Sogard. 2014. BOFFFFs: on the importance of conserving old-growth age structure in fishery populations. ICES Journal of Marine Science 71:171–2185.
Isaac, V. J., and M. C. Almeida. 2011. El consumo de pescado en la Amazonia brasileña. COPESCAALC Documento Ocasional No 13. FAO, Rome. Available at: https://www.fao.org/documents/card/en/c/f0b847ee-e6aa-5e4f-9d4b-6a0a3220e26f/.
Jacobi, C. M., F. Villamarin, J. V. Campos-Silva, T. Jardine, and W. E. Magnusson. 2020. Feeding of Arapaima sp.: integrating stomach contents and local ecological knowledge. Journal of Fish Biology 97:265–272.
Jézéquel, C., P. A. Tedesco, R. Bigorne, J. A. Maldonado-Ocampo, H. Ortega, M. Hidalgo, K. Martens, G. Torrente-Vilara,. Zuanon, A. Acosta, E. Agudelo, S B. Maure, D. A. Bastos, J. B. Gregory, F. G. Cabeceira, A. L. C. Canto, F. M. Carvajal-Vallejos, L. N. Carvalho, A. Cella-Ribeiro, R. Covain, C. Donascimiento, C. R. C. Dória, C. Duarte, E. J. G. Ferreira, A. V. Galuch, T. Giarrizzo, R. P. Leitão, J. G. Lundberg, M. Maldonado, J. I. Mojica, L. F. A. Montag, W. M. Ohara, T. H. S. Pires, M. Pouilly, S. Prada-Pedreros, L. J. de Queiroz, L. R. Py-Daniel, F. R. V. Ribeiro, R. R. Herrera, J. Sarmiento, L. M. Sousa, L. F. Stegmann, J. Valdiviezo-Rivera, F. Villa, T. Yunoki, and T. Oberdorff. 2020. A database of freshwater fish species of the Amazon basin. Scientific Data 7:96. https://doi.org/10.1038/s41597-020-0436-4.
Lennox, R. J, J. W. Brownscombe, S. J. Cooke, and A. J. Danylchuk. 2018. Post-release behaviour and survival of recreationally-angled arapaima (Arapaima cf. arapaima) assessed with accelerometer biologgers. Fisheries Research 207:197–203.
Lima-Junior, D. P., A. L. B. Magalhães, F. M. Pelicice, J. R. S. Vitule, V. M. Azevedo-Santos, M. L. Orsi, D. Simberloff, and A. A. Agostinho. 2018. Aquaculture expansion in Brazilian freshwaters against the Aichi Biodiversity Targets. Ambio 47:427–440.
Lombardo, U., J. Iriarte, L. Hilbert, J. Ruiz-Pérez, J. M. Capriles, and H. Veit. 2020. Early Holocene crop cultivation and landscape modification in Amazonia. Nature 581:190–193. DOI: 10.1038/s41586-020-2162-7.
Marková, J., R. Jerikho, Y. Wardiatno, M. M. Kamal, A. L. B. Magalhães, L. Bohatá, L. Kalous, and J. Patoka. 2020. Conservation paradox of giant arapaima Arapaima gigas (Schinz, 1822) (Pisces: Arapaimidae): endangered in its native range in Brazil and invasive in Indonesia. Knowledge & Management of Aquatic Ecosystems 421:47.
McGrath, D. G., L. Castello, O. T. Almeida, and G. M. Estupiñán. 2015. Market formalization, governance, and the integration of community fisheries in the Brazilian Amazon. Society and Natural Resources 28:513–529. https://doi.org/10.1080/08941920.2015.1014607.
Moran, E. 1993. Through Amazonian eyes: the human ecology of Amazonian populations. University of Iowa Press, Iowa City.
Nepstad, D., D. McGrath, C. Stickler, A. Alencar, A. Azevedo, B. Swette, T. Bezerra, M. DiGiano, J. Shimada, R. S. da Motta, E. Armijo, L. Castello, P. Brando, M. C. Hansen, M. McGrath-Horn, O. Carvalho, and L. Hess. 2014. Slowing Amazon deforestation through public policy and interventions in beef and soy supply chains. Science 344(6188):1118–1123. DOI: 10.1126/science.1248525.
Nobile, A. B., A. M. Cunico, J. R. S. Vitule, J. Queiroz, A. P. Vidotto-Magnoni, D. A. Z. Garcia, M. L. Orsi, F. P. Lima, A. A. Acosta, R. J. da Silva, F. D. do Prado, F. Porto-Foresti, H. Brandão, F. Foresti, C. Oliveira, and I. P. Ramos. 2020. Status and recommendations for sustainable freshwater aquaculture in Brazil. Reviews in Aquaculture 12:1495–1517.
Nogueira, F., M. Amaral, G. Malcher, N. Reis, M. A. D. Melo, I. Sampaio, P. S. Rêgo, and J. Araripe. 2020. The arapaima, an emblematic fishery resource: Genetic diversity and structure reveal the presence of an isolated population in Amapá. Hydrobiologia 847(15):3169–3183. https://doi.org/10.1007/s10750-020-04292-0.
Nuñez, J. 2012. Cultured aquatic species information programme: Arapaima gigas. Food and Agriculture Organization of the United Nations, Rome.
Núñez, J., F. Chu-Koo, M. Berland, L. Arévalo, O. Ribeyro, F. Duponchelle, and J. F. Renno. 2011. Reproductive success and fry production of the paiche or pirarucu, Arapaima gigas (Schinz), in the region of Iquitos, Perú. Aquaculture Research 42:815–822.
Ohs, C. L., J. E. Hill, S. E. Wright, H. M. Giddings, and A. L. Durland. 2021. Candidate species for Florida aquaculture: Arapaima Arapaima gigas. FA236. Institute of Food and Agricultural Sciences, University of Florida. https://doi.org/10.32473/edis-FA236-2021.
Ostrom, E. 1990. Governing the commons: the evolution of institutions for collective action. Cambridge University Press.
Oviedo, A. F. P., and M. Bursztyn. 2016. The fortune of the commons: participatory evaluation of small-scale fisheries in the Brazilian Amazon. Environmental Management 57:1009–d1023.
Oviedo, A. F. P., M. Bursztyn, and J. A. Drummond. 2015. Now under new administration: fishing agreements in the Brazilian Amazon floodplains. Ambiente & Sociedade 18(4):113–132.
Padial, A. A., A. A. Agostinho, V. M. Azevedo-Santos, F. A. Frehse, D. P. Lima-Junior, A. L. B. Magalhães, R. P. Mormul, F. M. Pelicice, L. A. V. Bezerra, M. L. Orsi, M. Petrere-Junior, and J. R. S. Vitule . 2017. The “Tilapia Law” encouraging nonnative fish threatens Amazonian River basins. Biodiversity and Conservation 26(1):243–246.
Pelicice, F. M., V. M. Azevedo-Santos, J. R. S. Vitule, M. L. Orsi, D. P. Lima-Junior, A. L. B. Magalhães, P. S. Pompeau, M. Petrere Jr., and A. A. Agostinho. 2017. Neotropical freshwater fishes imperiled by unsustainable policies. Fish and Fisheries 18:1119–1133.
Pelicice, F. M., and L. Castello. 2021. A political tsunami hits Amazon conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 31:1221–1229. https://doi.org/10.1002/aqc.3565.
Petersen, T. A., S. M. Brum, F. Rossoni, G. F. V. Silveira, and L. Castello. 2016. Recovery of Arapaima sp. populations by community-based management in floodplains of the Purus River, Amazon. Journal of Fish Biology 89:241–248. https://doi.org/10.1111/jfb.12968.
Prince, J. D. 2003. The barefoot ecologist goes fishing. Fish and Fisheries 4:359–371.
Reid, A. J., L. E. Eckert, J-F. Lane, N. Young, S. G. Hinch, C. T. Darimont, S. J. Cooke, N. C. Ban, and A. Marshall. 2021. “Two-eyed seeing”: an indigenous framework to transform fisheries research and management. Fish and Fisheries 22:243–261.
Reis, R. E., J. S. Albert, F. Di Dario, M. M. Mincarone, P. Petry, and L. A. Rocha. 2016. Fish biodiversity and conservation in South America. Journal of Fish Biology 89:12–47.
Roosevelt, A. C. 1999. Twelve thousand years of human-environment interaction in the Amazon floodplain. Pages 371–392 in C. Padoch, J. M. Ayres, M. Pinedo-Vasquez, and A. Henderson, editors, Várzea: diversity, conservation, and development of Amazonia’s whitewater floodplains. New York Botanical Garden Press, New York.
Sakaris, P. C., D. L. Buckmeier, N. G. Smith, and D. J. Daugherty. 2019. Daily age estimation reveals rapid growth of age-0 Alligator Gar in the wild. Journal of Applied Ichthyology 35:1218–1224.
Sautchuk, C. E. 2012. Cine-weapon: the poiesis of filming and fishing. Vibrant Virtual Brazilian Anthropology 9:406–430. DOI: 10.1590/S1809-43412012000200015.
Schaefer, F., W. Kloas, and S. Würtz. 2012. Arapaima: Candidate for intensive freshwater culture. Global Aquaculture Advocate website. Available at: https://www.globalseafood.org/advocate/arapaima-candidate-for-intensive-freshwater-culture/.
Schons, S. Z., G. Amacher, K. Cobourn, and C. Arantes. 2020. Benefits of community fisheries management to individual households in the floodplains of the Amazon River in Brazil. Ecological Economics 169:106531. https://doi.org/10.1016/j.ecolecon.2019.106531.
Schor, T., and G. S. Azenha. 2017. Ribeirinho food regimes, socioeconomic inclusion and unsustainable development of the Amazonian floodplain. EchoGéo [online] 41:15052. DOI: https://doi.org/10.4000/echogeo.15052.
Schwenke, K. L., and J. A. Buckel. 2008. Age, growth, and reproduction of Dolphinfish (Coryphaena hippurus) caught off the coast of North Carolina. Fishery Bulletin 106:82–92.
Sherman, V. R., H. Quan, W. Yang, R. O. Ritchie, and M. A. Meyers. 2017. A comparative study of piscine defense: the scales of Arapaima gigas, Latimeria chalumnae and Atractosteus spatula. Journal of the Mechanical Behavior of Biomedical Materials 73:1–16.
Sinovas, P., B. Price, E. King, A. Hinsley, and A. Pavitt. 2017. Wildlife trade in the Amazon countries: an analysis of trade in CITES listed species. Technical report prepared for the Amazon Regional Program (BMZ/DGIS/GIZ). UN Environment—World Conservation Monitoring Centre, Cambridge. Available at: https://www.cbd.int/doc/c/13f7/3a91/0b533d2c489c5e6ab06bc51f/sbstta-21-inf-08-en.pdf.
Smith, N. J. H. 1999. The Amazon River forest: a natural history of plants, animals, and people. Oxford University Press, New York.
Stewart, D. J. 2013a. A new species of Arapaima (Osteoglossomorpha: Osteoglossidae) from the Solimões River, Amazonas State, Brazil. Copeia 2013:470–476. doi: 10.1643/ci-12-017.
Stewart, D. J. 2013b. Re-description of Arapaima agassizii (Valenciennes), a rare fish from Brazil (Osteoglossomorpha: Osteoglossidae). Copeia 2013:38–51. doi: 10.1643/ci-12-013.
Torati, L. S., H. Migaud, M. K. Doherty, J. Siwy, W. Mullen, P. E. C. Mesquita, and A. Albalat. 2017. Comparative proteome and peptidome analysis of the cephalic fluid secreted by Arapaima gigas (Teleostei: Osteoglossidae) during and outside parental care. PloS One 12:e0186692.
Tregidgo, D. J., J. Barlowa, P. S. Pompeub, M.-D. A Rochac, and L. Parrya. 2017. Rainforest metropolis casts 1,000-km defaunation shadow. Proceedings of the National Academy of Sciences 114(32):8655–8865. https://doi.org/10.1073/pnas.1614499114.
Van der Sleen, P., and J. S. Albert. 2017. Field guide to the fishes of the Amazon, Orinoco, and Guianas. Princeton University Press, Princeton, NJ.
Viana, J. P., J. M. B. Damasceno, L. Castello, and W. G. R. Crampton. 2004. Community management of fishery resources in the Mamiraua Sustainable Development Reserve, Brazil. Pages 139–154 in K. M. Silvius, R. E. Bodmer, and J. M. V. Fragoso, editors, People in nature: wildlife conservation in South and Central America. Columbia University Press, New York.
Walker, P. 2009. Dinosaur DAD and enlightened EDD: engaging people earlier is better. Environmentalist 71:12–13.
Watson, L. C., and D.J . Stewart. 2020. Growth and mortality of the giant Arapaima in Guyana: implications for recovery of an over-exploited population. Fisheries Research 231:105692. https://doi.org/10.1016/j.fishres.2020.105692.
Watson, L. C., D. J. Stewart, K. Clifford, L. Castello, D. Jafferally, S. James, Z. Norman, and G. G. Watkins. 2021. Recovery, conservation status, and environmental effects on Arapaima populations in Guyana. Aquatic Conservation: Marine and Freshwater Ecosystems 31:2533–2546. https://doi.org/10.1002/aqc.3628.
Watson, L. C., D.J . Stewart, and A. M. Kretzer. 2016. Genetic diversity and population structure of the threatened giant Arapaima in southwestern Guyana: implications for their conservation. Copeia 104:864–872. https://doi.org/10.1643/cg-15-293.
Watson, L. C., D. J. Stewart, and M. A. Teece. 2013. Trophic ecology of Arapaima in Guyana: giant omnivores in neotropical floodplains. Neotropical Ichthyology 11:341–349. doi: 10.1590/s1679-62252013000200012.
World Bank. 2012. Hidden harvest: the global contribution of capture fisheries. World Bank, Washington, D.C. https://openknowledge.worldbank.org/handle/10986/11873. License: CC BY 3.0 IGO.
Yang, W., H. Quan, M. A. Myers, and R. O. Ritchie. 2019. Arapaima fish scale: one of the toughest flexible biological materials. Matter 1:1557–1566.
Narrow strip of land with sea on either side, forming a link between two larger areas of land
Starch or flour obtained from the root of cassava, a tropical tree
Large order of freshwater fish that occur in Africa, South America, and Central America
Citrus-and-cheese-flavored fruit from a native tree of Brazil's highlands
Restricted to a particular function or mode of life
Treated as insignificant or peripheral
Relating to or denoting the zone of the seashore between high- and low-water marks, or the zone near a lake shore with rooted vegetation
Recently hatched fish larva that is still too immature to achieve motility and relies on yolk sac for nutrition
Cell in an ovary which may undergo meiotic division to form an ovum
Branch of science concerned with classification of organisms
Advocate for or member of a technically skilled elite
Relating to the ear or the sense of hearing

