14 Menhaden and Forage Fish Management
Learning Objectives
- Describe the historical development of Atlantic Menhaden fishing.
- Summarize key characteristics of life history and ecological role of Atlantic Menhaden.
- Articulate the special challenges of managing fisheries on small, pelagic forage fish that are low on the food chain and make them vulnerable to both environmental change and overfishing.
- Highlight the false assumptions of single-species management for maximum sustainable yield.
- Explain the benefits of adopting a fishery policy that uses ecological reference points.
- Describe value preferences of key stakeholders in the Atlantic Menhaden fisheries.
14.1 Early Lessons Learned from Menhaden Fishing
Indigenous people along the Atlantic Coast and early European colonists relied heavily on abundant Atlantic Menhaden (Brevoortia tyrannus) (Figure 14.1). At least 30 popular names are used to describe the menhaden. In Maine and Massachusetts, the names “pogy” and “hardhead” are used. In New York and New Jersey, the name “mossbunker” (with a variety of spellings) is common, whereas in Delaware and Chesapeake Bay, the names “old-wife,” “alewife,” “greentail,” and “bug-fish” are also used. In the Carolinas, they may be referred to as “fat-back,” “yellow-tail,” or “yellow-tailed shad.” “Pogy” and “menhaden” were likely derived from Native American dialects of New England. Somehow, the name “Munnawhatteaûg,” which means “fertilizer” or “manure,” was corrupted to menhaden. As early as 1661, the name “mossbanker” was in use based on Jacob Steendam’s poem “Praise of New Netherland”:
The black and rock-fish, herring, mackerel,
The haddock, mossbanker, and roach, which fill
The nets to loathing; and so many, all
Cannot be eaten.
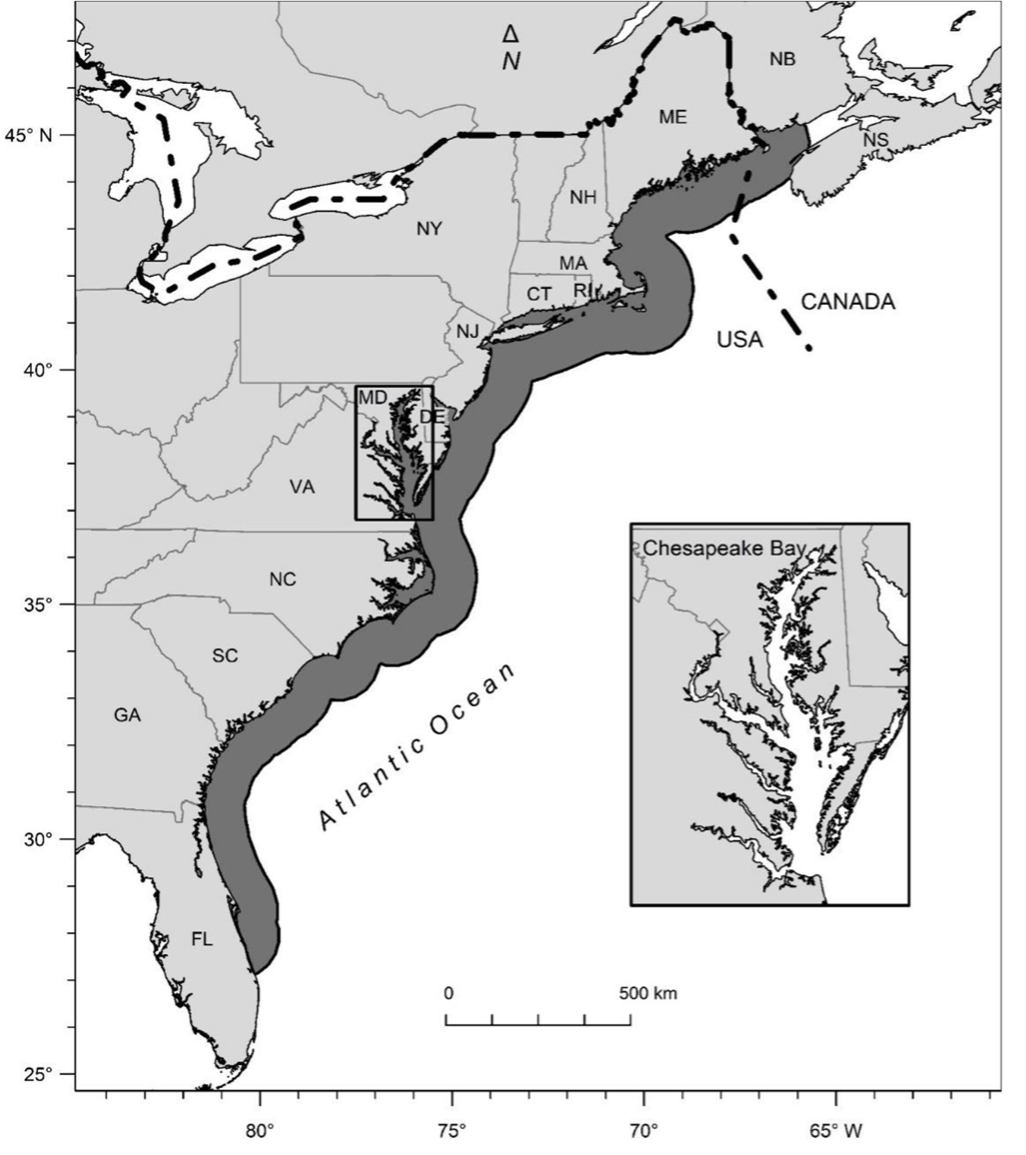
The history of menhaden management reveals challenges of breaking from long-standing traditions in fisheries management. The Atlantic Menhaden was labeled as the “most important fish in the sea” by author and historian Bruce Franklin because of its utility to Native Americans and European colonists in the Atlantic coastal regions (Figure 14.2). Native Americans instructed early colonists how to plant their crops with abundant menhaden as manure. Later, colonists often plowed excess harvest of menhaden in the soil of farms along the shore, until new products utilizing them were discovered, initiating a large industrial fishery.
Soon colonists realized the value of the menhaden as a baitfish, as well as an important source of oil, manure (guano), and fish meal. From 1850 to 1870, numerous factories from Maine to North Carolina began to manufacture oil from menhaden. Soon the yield of oil from menhaden would exceed yield of oil from whaling. Fish scrap, or waste after oil was pressed out of the fish, was sold as manure for fertilizer.
A large industry based on menhaden developed along the Atlantic Coast in the late 19th century. Although there were no fishing regulations at the time, the U.S. Oil and Guano Association monitored companies and total catch. By 1876, menhaden yields were 462 million pounds and valued at $1,657,790 (Goode 1880). At the time, Marshall McDonald, Commissioner of Fisheries for Virginia, wrote that “this industry is yet in its infancy.” The early landings exceeded the volume of recent menhaden landings from both the Gulf of Mexico and Atlantic Coast of 1,581,578 pounds, valued at $161 million (National Marine Fisheries Service 2020). Currently, the landings of menhaden are second in volume behind Alaska Pollock, and they are higher than landings of salmon and cod combined. Value of menhaden landings ranks 10th after high-valued seafoods. Estimates from 1878 indicated that 279 sail vessels caught nearly 900 million menhaden, which were processed by 56 factories and yielded 4 million barrels of fish oil and 30,000 tons of guano (Goode 1880).
The reduction factories that were once widespread along the Atlantic Coast were smelly operations. In the 20th century, many states banned them in the interest of coastal development and odor abatement. The only states on the Atlantic Coast that still permit reduction fishing are North Carolina and Virginia. Since 2005, all harvested menhaden are processed at one facility at Reedville, Virginia, owned by Omega Protein, a subsidiary of Canadian-based Cooke Aquaculture. While there are four species of menhaden, the most abundant are the Atlantic Menhaden, distributed from Maine to Florida, and the Gulf Menhaden (Brevoortia patronus), distributed in the Gulf of Mexico. Two other species are distributed in the Gulf of Mexico and the Atlantic Coast of Florida.
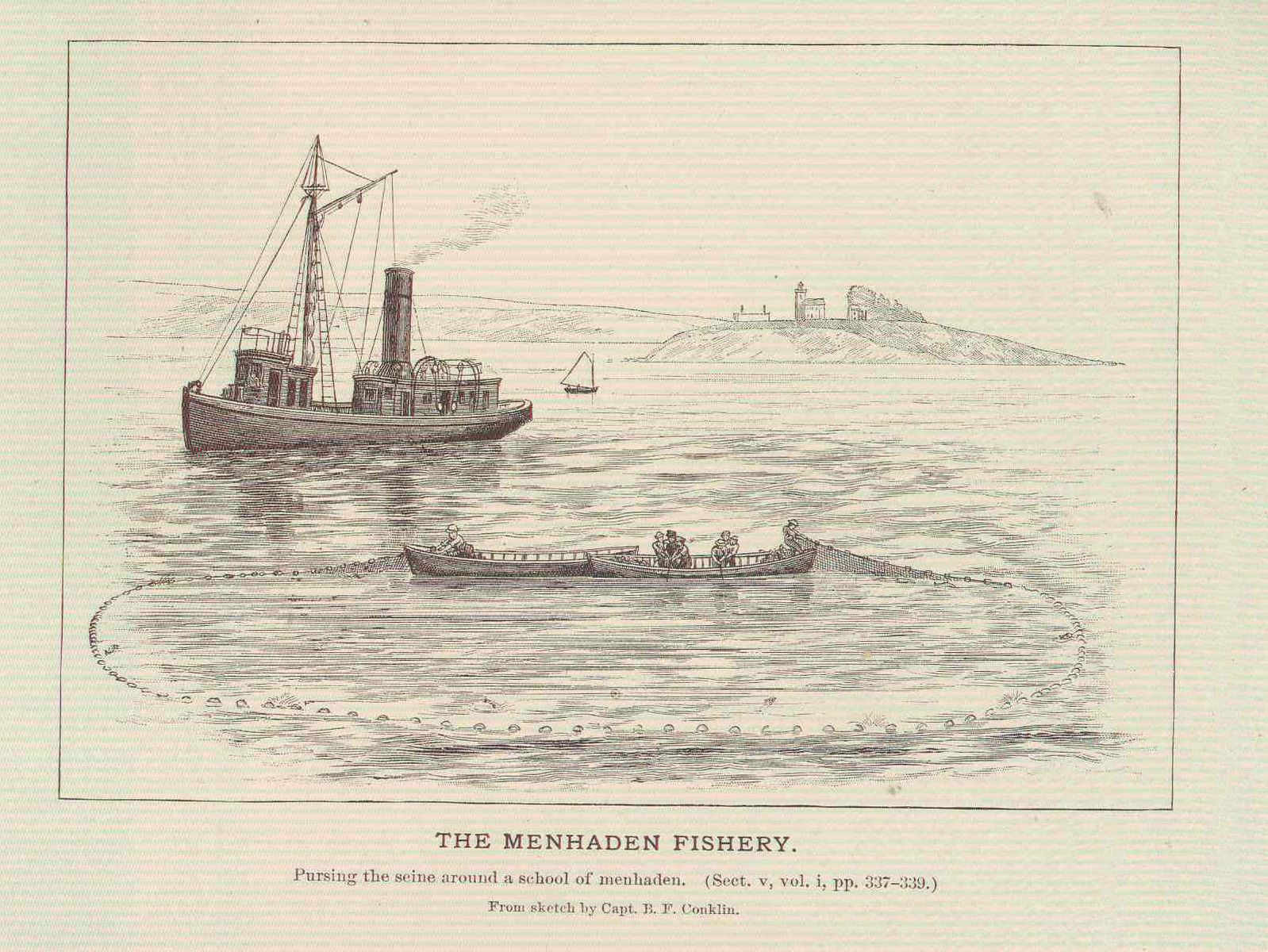
Early commercial vessels learned that the purse seine is more effective than any other fishing apparatus for catching menhaden (Figure 14.3). A school of almost any size may be surrounded by the net, resulting in a catch composed entirely of menhaden. Factory fleets exclusively rely on the purse seine. Early versions extended 90 to 180 feet below the surface and from 800 to 1,500 feet in length (Goode 1880); some boats carried both long and short purse seines to adapt to the size of menhaden schools.
14.2 Life History of Menhaden
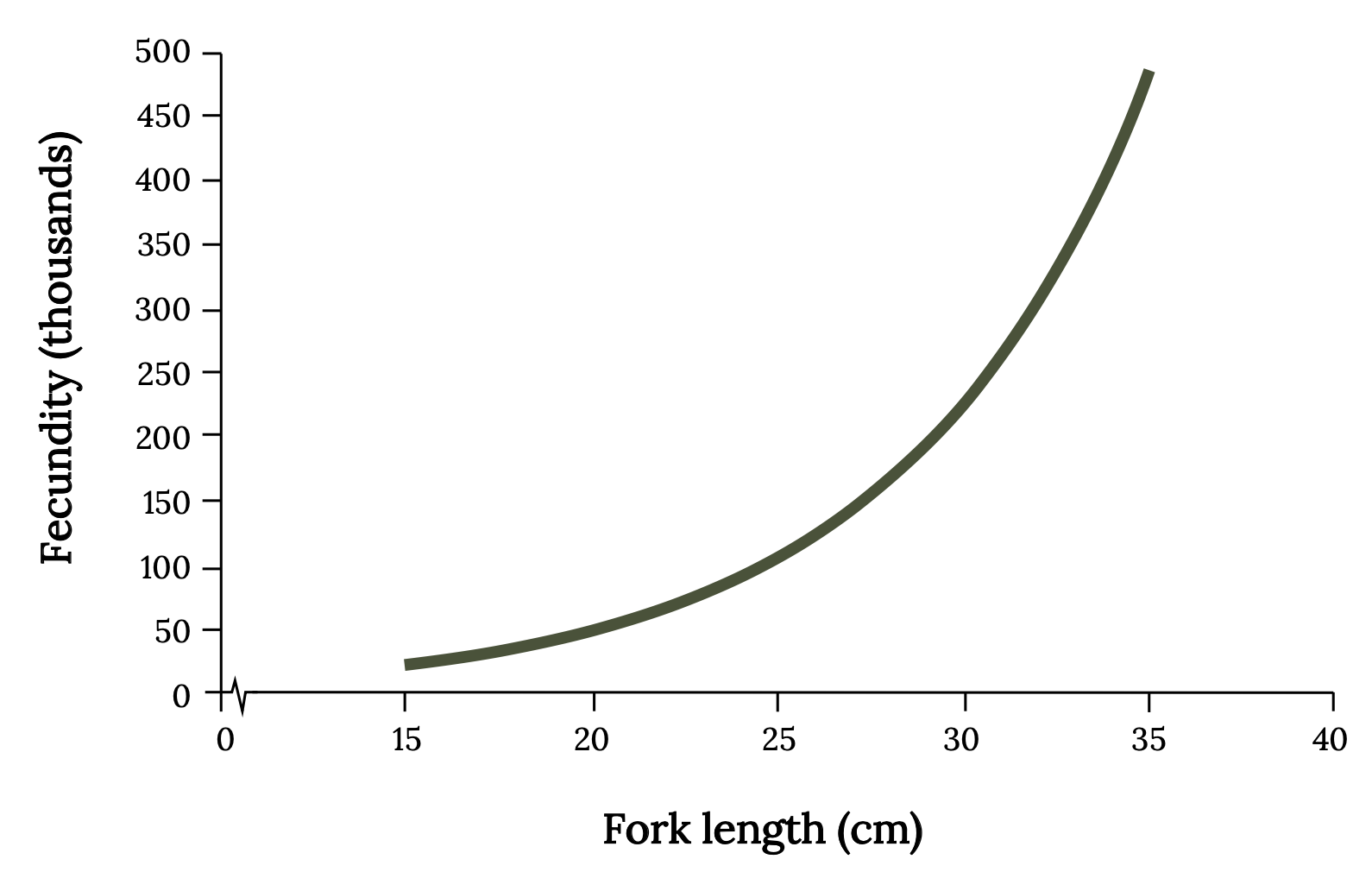
Menhaden have an opportunistic life history strategy, characterized by many small offspring, small body size, rapid growth, early maturity, and short life span relative to other fish. Menhaden spawn offshore from fall through spring, with peak spawning in fall and winter. Atlantic Menhaden exhibit indeterminate batch spawning, which means that the eggs in the female continue to mature during the spawning season for release in later spawns. Mature menhaden may spawn up to 10 times per season. The fertilized eggs are small (~1.6 mm), and mature females may produce about 240 eggs per gram of body weight, and sometimes as many as 700. Total fecundity is difficult to know because eggs continue to mature during a long spawning season, fish spawn multiple times, and the number of eggs is strongly influenced by menhaden size (Figure 14.4). Because of variability in spawning time and growth, some Atlantic Menhaden may become sexually mature by age 1 (~20–24 cm), while most become fully mature at age 4. In the absence of fishing, Atlantic Menhaden can live 10 to 12 years and attain a length of 38 cm (i.e., 15 inches). The larger menhaden are big enough to become the preferred prey of large Striped Bass and Ospreys.
Eggs are buoyant in sea water and drift with currents. After hatching, the larval (~1–2 cm) and juvenile (~3.0–3.5 cm) Atlantic Menhaden drift with currents, grow rapidly, and return to bays and estuaries, which are important nursery areas. After less than one year in bays and estuaries, most of the juveniles return to sea. Atlantic Menhaden grow rapidly in the first year, and the smallest menhaden caught in the fishery will include some age-0 fish. Growth rates are also strongly influenced by densities and water temperature, and high landings in one year may permit faster growth the following year. In addition, wind and climate conditions have a large influence on growth (Midway et al. 2020). Consequently, environmental conditions that lead to large year classes or year-class failure may occur at a variety of times and locations.
The life history characteristics result in highly variable recruitment where there may be no discernible relationship between abundance of spawners and the resulting recruitment of offspring (Schaaf and Huntsman 1972). Further, the biological reference points used to adjust fishing quotas are also highly uncertain (Schueller and Williams 2017). This means that a harvest quota that was at one time sustainable may lead to the collapse of a fishery after climatic conditions become less favorable for reproduction and growth.
Many other small, pelagic fish, such as sardine and anchovy, also support large coastal fisheries that are known to show dramatic fluctuations in abundance. High levels of commercial fishing on these small pelagic fish increase the chance for collapse (MacCall et al. 2016). Well-documented and widespread collapses of Pacific Sardine (Sardinops sagax) occurred in the 1940s and again from 2008 to the present. Recruitment in other clupeiform (herring-life) fish is driven largely by environmental variation or climatic regime shifts (Essington et al. 2015). For example, Atlantic Menhaden recruitment is strongly influenced by sea surface temperature (Deyle et al. 2018), which leads to high catch variability in the youngest adult age classes and variability in the commercial harvest. Consequently, aspects of the life history of Atlantic Menhaden may translate to poor prediction of trends with classical fisheries models (Szuwalski and Thorson 2017).
14.3 Ecological Role of Menhaden
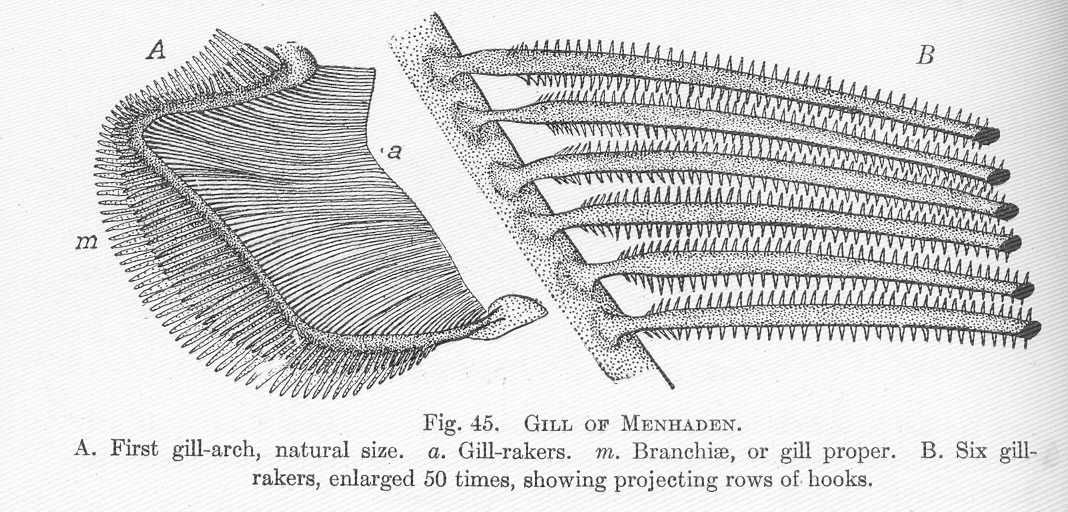
Atlantic Menhaden swim through the water column with their mouths wide open, thereby trapping food particles on the gill rakers. Gill structures of the Atlantic Menhaden (Figure 14.5) create an effective sieve for efficient filter feeding. Both plant and animal plankton are consumed by Atlantic Menhaden. When smaller, they feed primarily on phytoplankton; however, they shift their diet to primarily consume zooplankton as they grow. By one oft-cited estimate, Atlantic Menhaden are capable of filtering 23–27 liters of water per minute (Peck 1894). Consequently, they manage the large algal bloom occurrences in the bay because they eat vast quantities of phytoplankton, thereby reducing concentrations of nutrients. Watch this video to observe feeding behavior (Filter-Feeding Menhaden Caught on Camera, https://www.youtube.com/watch?v=WhprcLcGGBs).
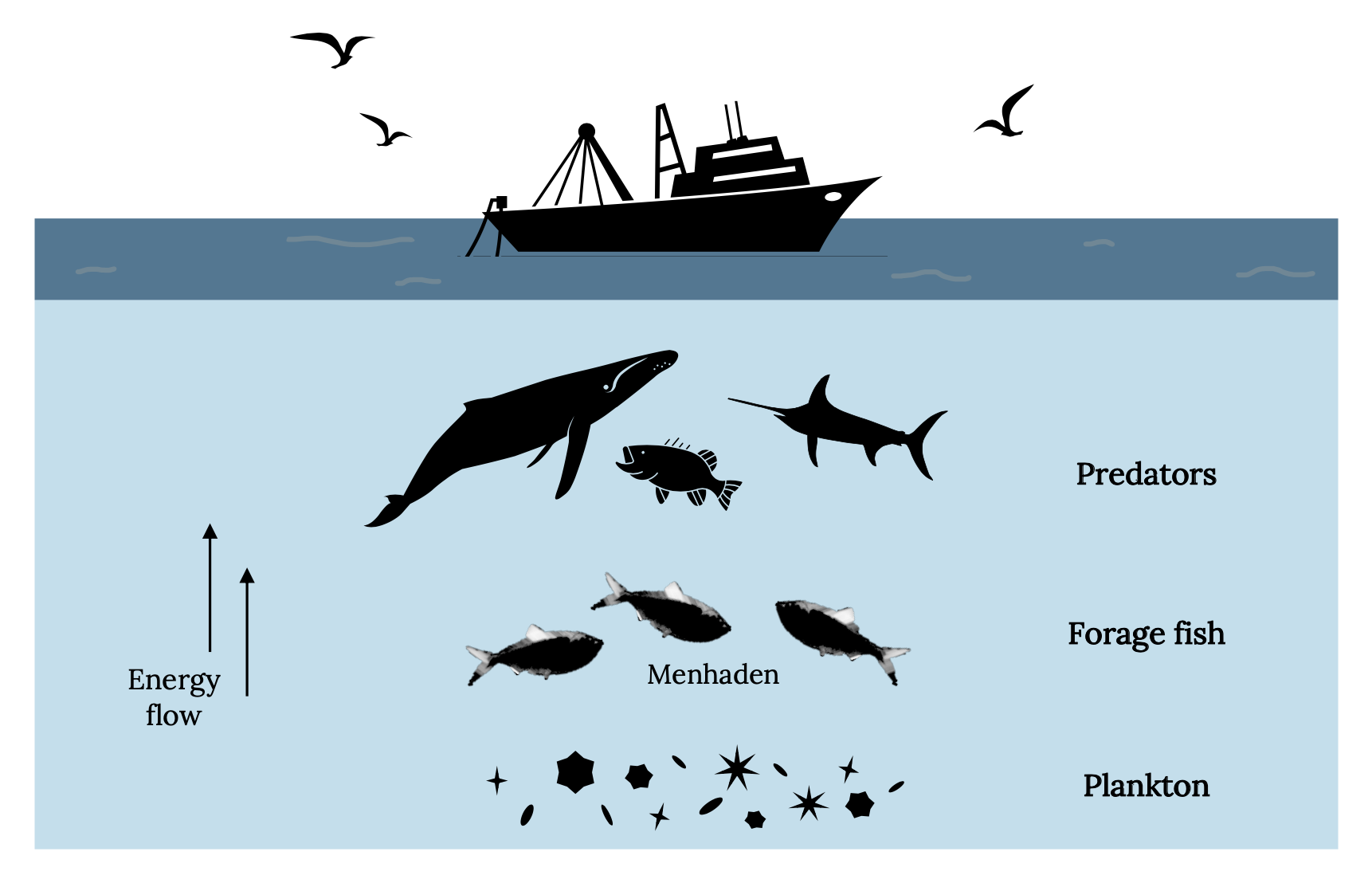
All carnivorous sea mammals, fish, and seabirds are potential predators of menhaden. Consequently, whales, dolphins, sharks, tuna, swordfish, bonito, Striped Bass, Weakfish, Tarpon, and bluefish likely consume large numbers of menhaden. Atlantic Menhaden are an important link between the plankton and numerous carnivorous predators (Figure 14.6).
The feeding behavior of predators on large schools of menhaden provides an impressive display, which is easily seen from a distance. Consider the sharks entering a large school of menhaden captured with this drone-mounted video camera off the coast of New York Hampton in 2017.
Fish predators force the menhaden to move near the surface, where they are more easily eaten by piscivorous birds. Ospreys, along with Bald Eagles, feed on menhaden, which are easily captured from the water surface (Figure 14.7). Chesapeake Bay once supported the largest concentration of breeding Ospreys in the world, and breeding populations of both Bald Eagles and Ospreys have been recovering since DDT was banned. The high lipid content of Atlantic Menhaden nourishes Ospreys, which breed throughout the coastal waters of the mid-Atlantic and northeastern United States (Spitzer and Poole 1980). One study indicated that Atlantic Menhaden comprised 24% of fish brought to Osprey nestlings in lower estuarine sites (Glass and Watts 2009).

Until recently, few investigations have quantitatively linked abundance of menhaden predators to their abundance. However, after regulation of the Atlantic Menhaden fishery, the fish rebounded and expanded back into their historic range while Humpback Whales were recovering after the ban on whaling in 1955 (Stevick et al. 2003; Brown et al. 2018; Lucca and Warren 2019). Observations by Gotham Whale (GothamWhale.org), a New York City–based whale research organization, have shown an increase in whale sightings in the New York–New Jersey Bight in the last 10 years (Brown et al. 2022). Watch this video of pilot whales and Humpback Whales feeding on pods of menhaden: Menhaden Conservation Works, New York, by Timothy Del Grosso, https://vimeo.com/239293026.
Black Skimmer, a widely distributed tern-like bird, is uniquely adapted to feeding on surface-dwelling fish. As the bird flies low, its long lower mandible plows the water, snapping the bill shut when it contacts a fish. Black Skimmers consume many species of small fish, and Atlantic Menhaden makes up one-fourth of its diet (Gordon et al. 2000). Watch this video: Black Skimmers–003, 2007, https://www.youtube.com/watch?v=7USpTc6MUoc. Populations of Black Skimmers have declined 86% between 1944 and 2015, leading to its listing as a Species of High Concern for conservation.
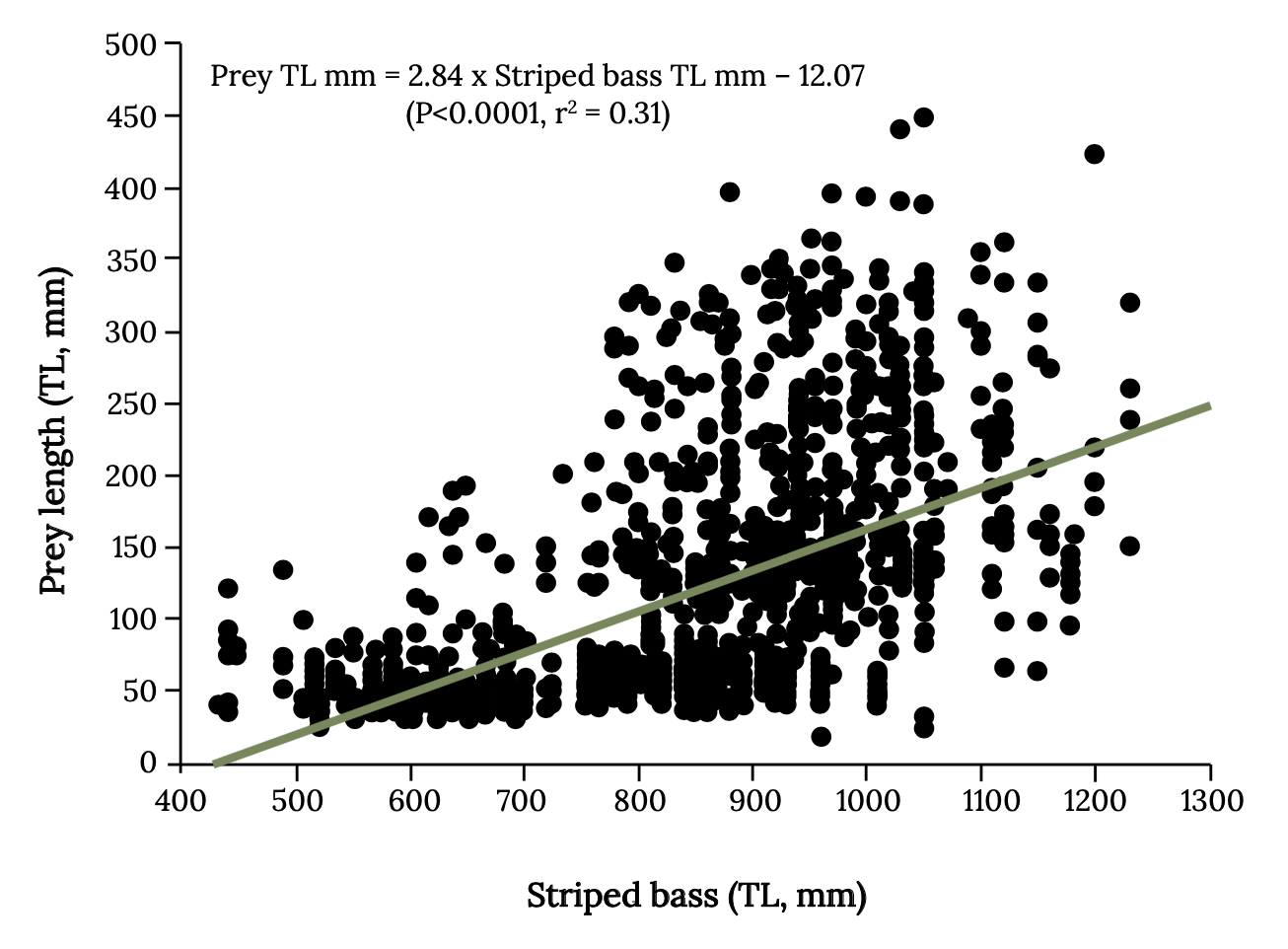
Strong overlap often occurs with size of forage fish eaten by birds, such as cormorants, boobies, pelicans, and those caught by commercial fishing (Pikitch et al. 2012, 2014, 2018). However, the impact that fishing on forage fish has on their predators will depend on number and types of alternative prey and the size overlap between fish taken by fishing and predators (Hilborn et al. 2017). For example, there is strong overlap with size of menhaden harvested and size eaten by Atlantic Bluefin Tuna. Striped Bass eat a wider range of menhaden sizes, and the larger Striped Bass can eat larger menhaden, which are targeted by commercial fishing (Walter et al. 2003; Overton et al. 2008; Figure 14.8). Watch this video to see Bluefin Tuna feeding on menhaden: Bluefin Tuna Near Shore Attacking Menhaden 2020, https://www.youtube.com/watch?v=e-0JAodI2A0.
Question to ponder:
What considerations are most important when setting Atlantic Menhaden harvest regulations?
14.4 Industrial Fishing, Marine Pelagic Fish, and Menhaden
The industrial menhaden fishery is an example of what is happening in many parts of the world in fisheries that target small pelagic forage fish. Many products are produced from small pelagic forage fish, including canned anchovy and sardine, fish sauce, moisturizers, human health supplements, bait, fish oil, and fish meal. Globally, these marine fisheries are significant sources of livelihood, with over three-fourths of the production coming from developing countries. Fisheries for marine pelagic fish often have low levels of bycatch and greenhouse gas emissions because of the schooling behavior of fish and the use of purse seines that target them. However, some of these pelagic fisheries also take squid, seabirds, mammals, and carnivorous fish, raising concerns from conservationists. Of further concern, demand for small pelagic fish is likely to increase in the future for use in aquaculture feeds (Merino et al. 2010).
Anchovy and sardine, which make up 52% of landings of marine pelagic fish, share a similar life history pattern with Atlantic Menhaden. They produce numerous small offspring, reach a small body size, grow rapidly, mature early, and live a short life. Like menhaden, anchovy and sardine eat phytoplankton and zooplankton at or near the base of the food web by filtering particles or biting individual particles. Sardine and anchovy also have extensive coastwide migrations. These traits mean that marine pelagic forage fish show speedy and sometimes dramatic reactions to environmental change. Furthermore, overfishing and climate change in combination may drive collapse of anchovy and sardine fisheries (Checkley et al. 2017; Izquierdo-Peña et al. 2020).

Because Atlantic Menhaden undergo extensive migrations and are mostly harvested from inshore (state) waters, their management is coordinated through the Atlantic States Marine Fisheries Commission (ASMFC), a deliberative body of fisheries management agencies from the Atlantic Coast states. After Maryland banned the use of purse seines to harvest Atlantic Menhaden in 1931, Virginia and North Carolina were the only states to permit reduction purse-seine fishing. This fishery is concentrated in Virginia waters of Chesapeake Bay and offshore to stay close to the only surviving menhaden reduction plant in Reedville. Large purse seines are used to harvest menhaden for reduction to fish meal to oil (Figure 14.9). Smaller purse-seine rigs, called “snapper rigs,” are used for capture of menhaden for bait. In 1999, the lower Chesapeake Bay was the center of the Atlantic Menhaden fishery, with the bay, Virginia’s eastern shore, and Virginia Beach accounting for 67% of the total harvest of Virginia and North Carolina fleets (Smith 1999).
To assist ship captains in locating schools of menhaden, an airplane pilot in a spotter plane directs the ship’s two smaller purse boats, whose mission it is to trap the fish in an ever-tightening net. A hydraulic rig lifts the net to bring the catch closer to the surface, where a large vacuum hose sucks the fish into the ship’s hold. The crew’s pay is determined by the catch, so fishing crews work from Sunday night to Friday night. The oily catch is unloaded in Reedville each night, after which the crew returns to fishing grounds to catch more menhaden.
14.5 Demand for Products From Small Marine Pelagic Fish
Small marine pelagic fish, often herring or sardine, consume and process marine algae and incorporate omega-3 fatty acids in their bodies. Foods that are high in omega-3 fatty acids are essential for humans because these fatty acids cannot be synthesized in the body. Therefore, they must be consumed in the diet. Omega -3 fatty acids have many effects on the heart and blood vessels of humans, including reduction in triglycerides, irregular heartbeat, arterial plaque, and blood pressure. Therefore, their health benefits of omega-3 fatty acids have been promoted for heart health in patients with coronary heart disease to reduce the risk of heart attacks (Manson et al. 2020). More recent studies focus on the potential influence of omega-3 fatty acid consumption on cancer, depression, inflammation, cognitive decline, and ADHD (Arellanes et al. 2020).
Demand for small pelagic fish is likely to increase to meet growing demands for bait, aquaculture, and fish oil supplements. Forage fish are captured and sold as bait for sportfishing and crab and lobster traps. Global demand for use of fish meal in aquaculture feeds is rising dramatically, and fish oil is a growing global industry (Merino et al. 2010; World Bank 2013). In 1997, the Food and Drug Administration approved the use of refined menhaden oil for use in foods and supplements. Omega Protein, Inc., which operates the only marine oil refinery in the United States, produces several grades of refined menhaden oil.
Future demands for menhaden soluble fats, oil, and meal, while expected to be higher, are uncertain for several reasons. First, because of rising fish meal costs, feed industries are replacing fish meal with fermented soy and soybean protein concentrates. Globally, a surplus of soy depresses the demand for and price of fish meal. Second, similar products can be naturally derived from other sources. For example, marine algae chorella and spirulina, which can be cultured, are also high in omega-3 fatty acids, minerals, and antioxidants. Similarly, krill, flax, soybeans, nuts, and other plants also contain high levels. Some food companies are working to create a plant-based oil, LatitudeTM, that is high in omega-3. Finally, since the use of menhaden to produce omega-3 supplements is classified by the FDA as food, there is less regulatory oversight, there are no clinical trials, and supplements may contain harmful levels of mercury, PCBs, and dioxins (Hong et al. 2015; Sherratt et al. 2020).
14.6 Menhaden Population Dynamics
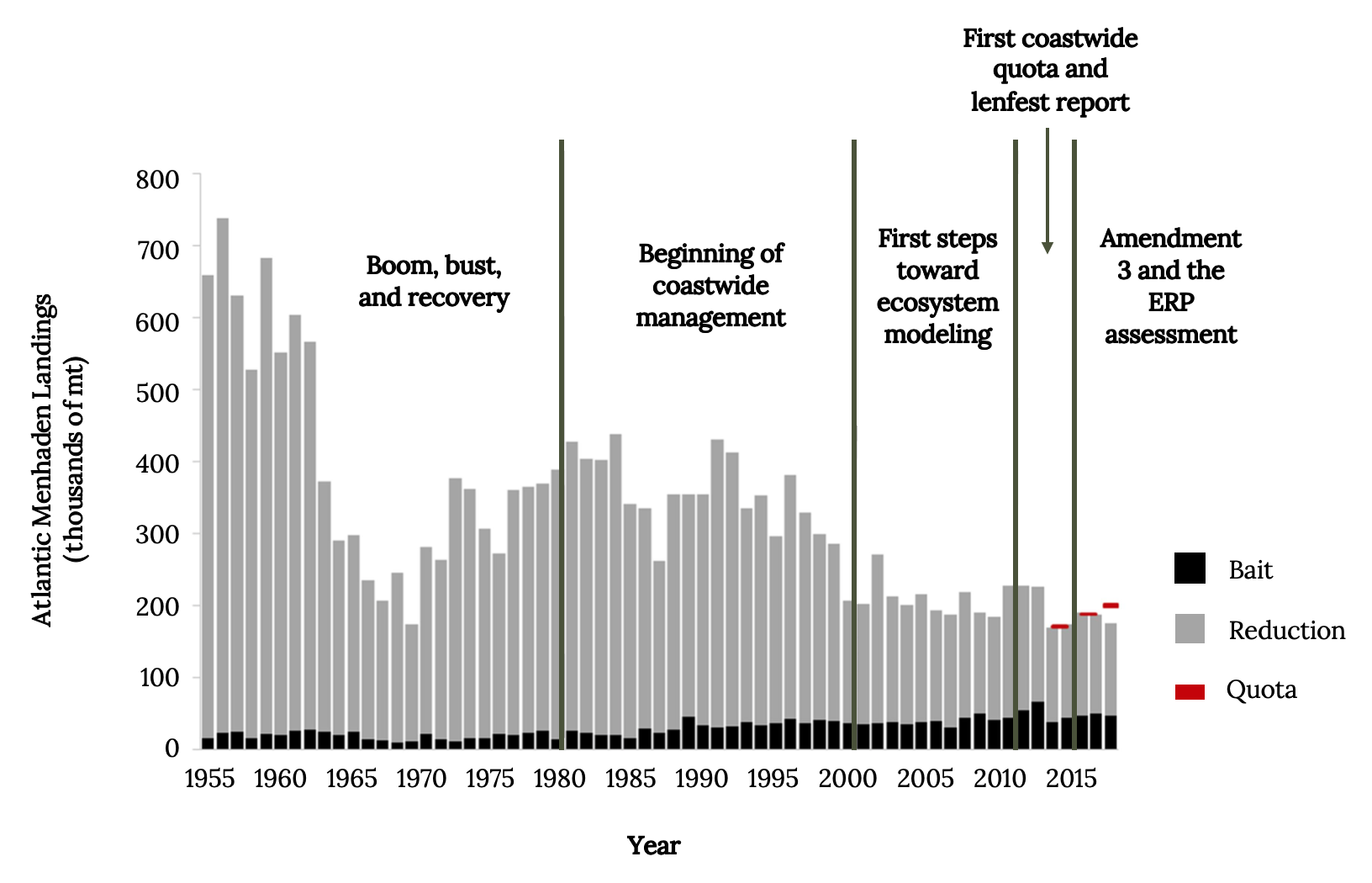
By 1876, menhaden yields already exceeded 200,000 metric tons (Figure 14.10). After World War II, menhaden fisheries went through a boom, bust, and recovery, which forced coastwide coordination of harvest quotas in the 1980s and 1990s.
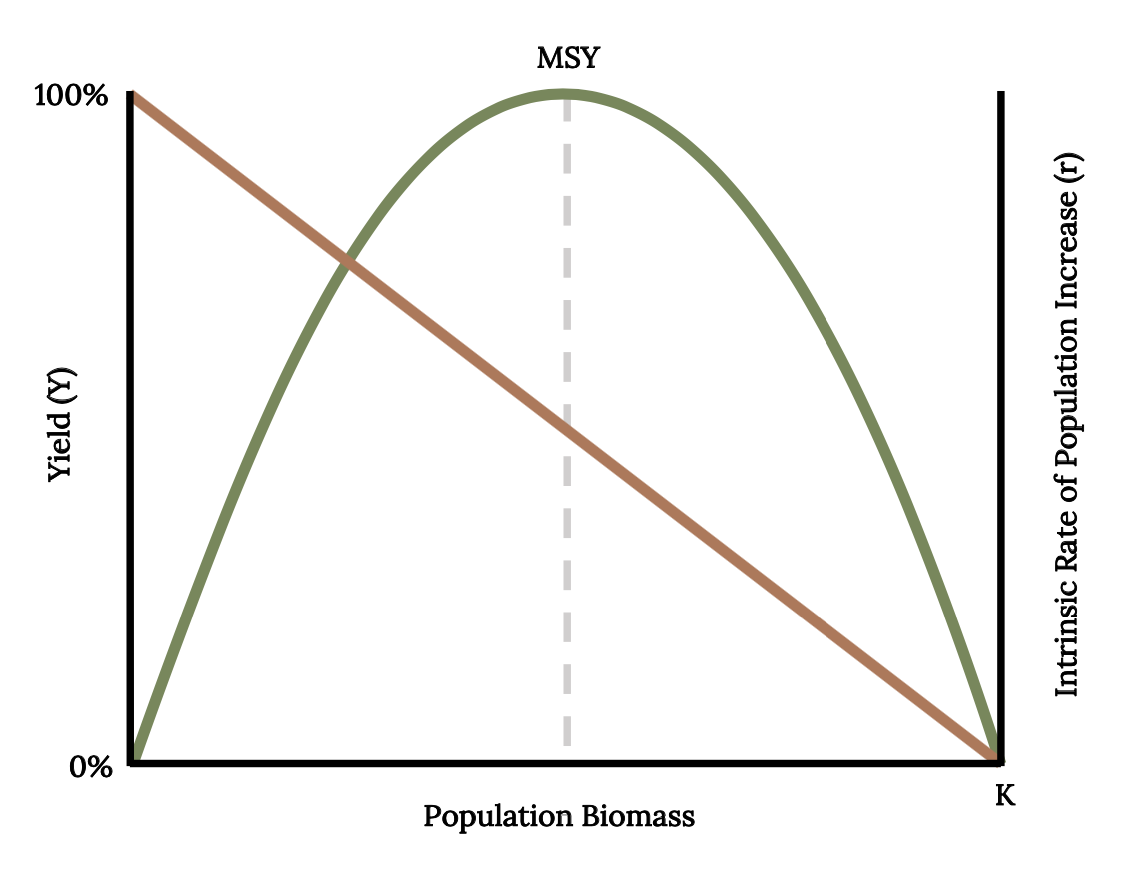
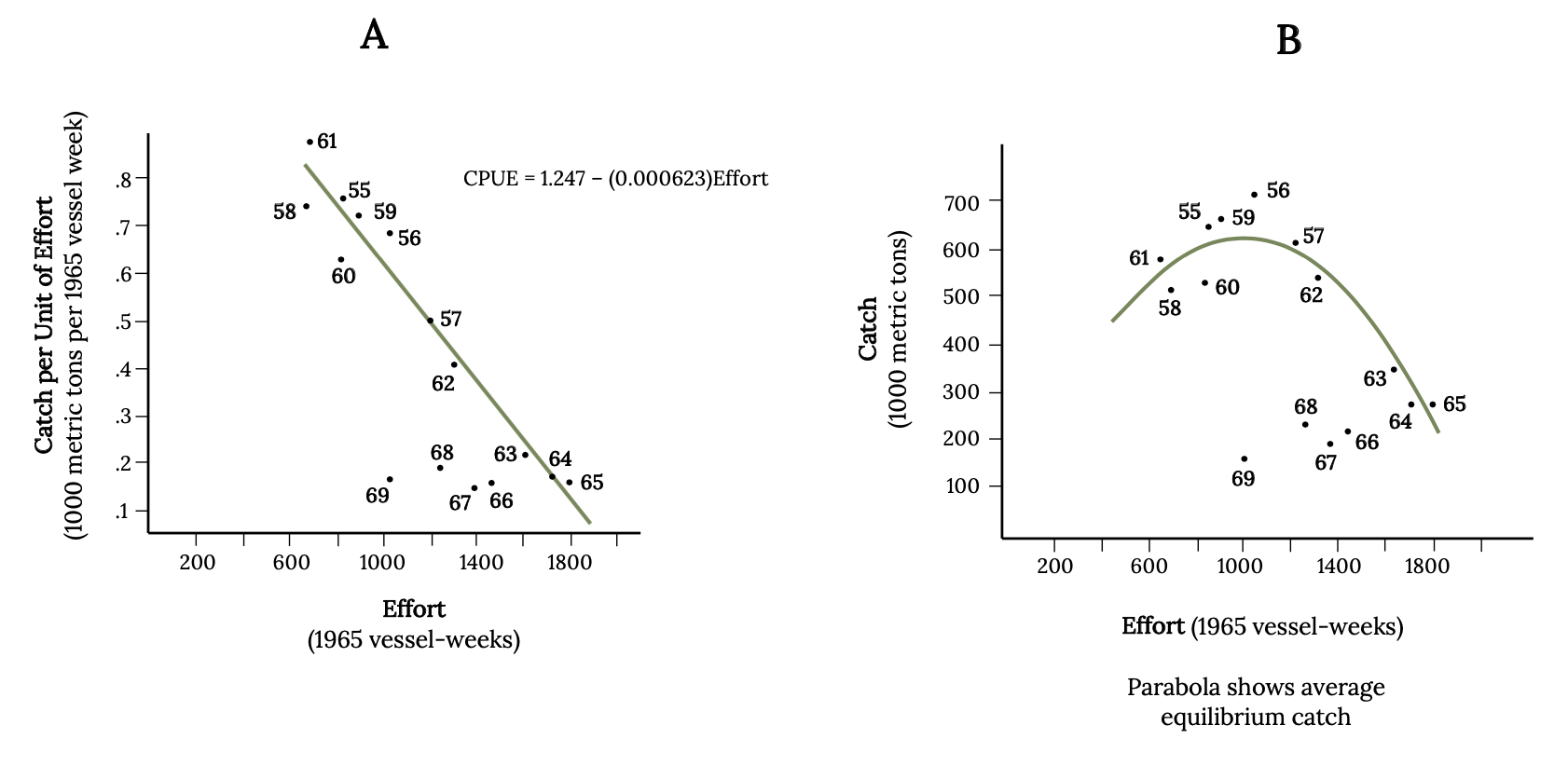
Early studies of population dynamics of Atlantic Menhaden applied single species population analysis tools, such as equilibrium yield and stock recruitment. With these mathematical tools, scientists can predict, theoretically, the largest catch that can be taken from a species’ stock over an indefinite period (Finley 2011). These models predict a dome-shaped relationship between long-term average yield, or equilibrium yield (Y), and population biomass (Figure 14.11). This result is because the rate of increase (r) declines in a linear manner as population approaches carrying capacity. Fisheries managers monitor catch per unit effort as a primary index of abundance because abundance is difficult to quantify. From the 1950s and through the 1960s, catch per unit effort decreased as fishing effort increased, as was expected (Figure 14.12 A; Schaff and Huntsman 1972). During the 1950s and 1960s, a dome-shaped relationship was evident from the scatterplot of the data (Figure 14.12 B). Since 1966, the data points diverged from the expected dome-shaped relationship between catch and effort to a linear relationship between catch per unit effort and fishing effort (Figure 14.12).
This linear decline in catch per unit effort was not reversed with reductions in fishing. Examine the data scatter, which shows how the reduction in effort after 1965 results in similar measures of catch per unit effort and total catch (Figure 14.12). There is a time lag in the response of menhaden populations to fishing reductions, related to their life history as well as changes in environmental conditions and/or predator abundance, which may also influence dynamics of the fish.
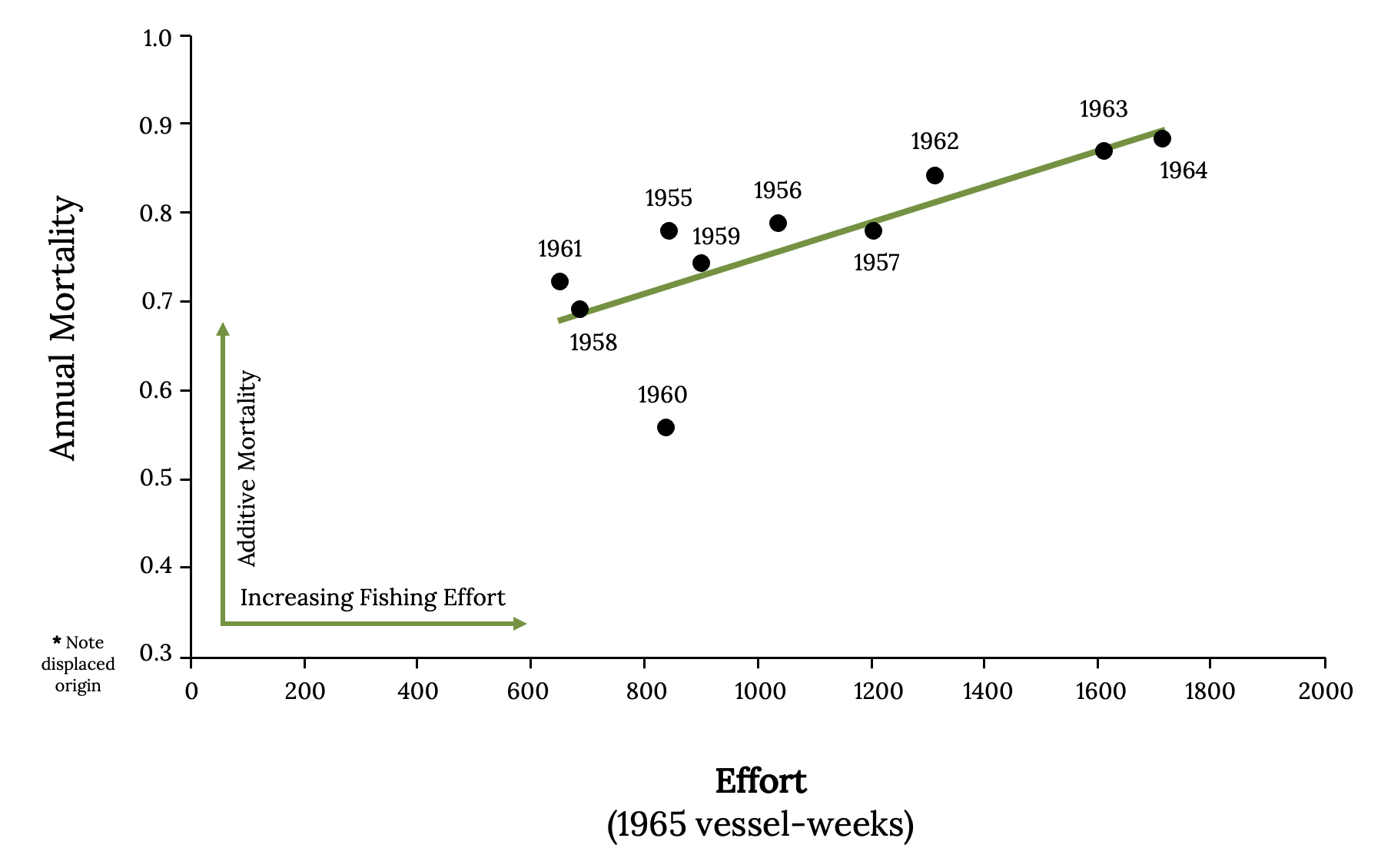
Without fishing, the mortality of Atlantic Menhaden is about 25% per year. However, with typical levels of fishing observed, mortality was between 65 to 85% per year (Figure 14.13). Predicting the population dynamics and estimating mortality of Atlantic Menhaden are also complicated by movements along the coast. One tagging study indicated that during May and June, an estimated 91% of Atlantic Menhaden from North and South Carolina moved northward. In the winter, an estimated 55% of the sample tagged north of Chesapeake Bay moved southward to the bay and North and South Carolina (Liljestrand et al. 2019). Therefore, it is difficult to measure abundance of a mobile population.
If fishing mortality gets too high, few fish in the largest size classes will survive to reproduce, and biomass of spawning fish will decrease. The first management plan was developed in response to major changes in the fishery efficiency at capturing menhaden, enhanced processing capacity, as well as the development of new markets for products. Recognizing that seasons and mesh size restrictions had not prevented decline in menhaden, the first plan focused on determining the appropriate age to first harvest them (ASMFC 1981). Only later did plans estimate target and threshold levels used to determine if quotas should be adjusted (ASMFC 1999).
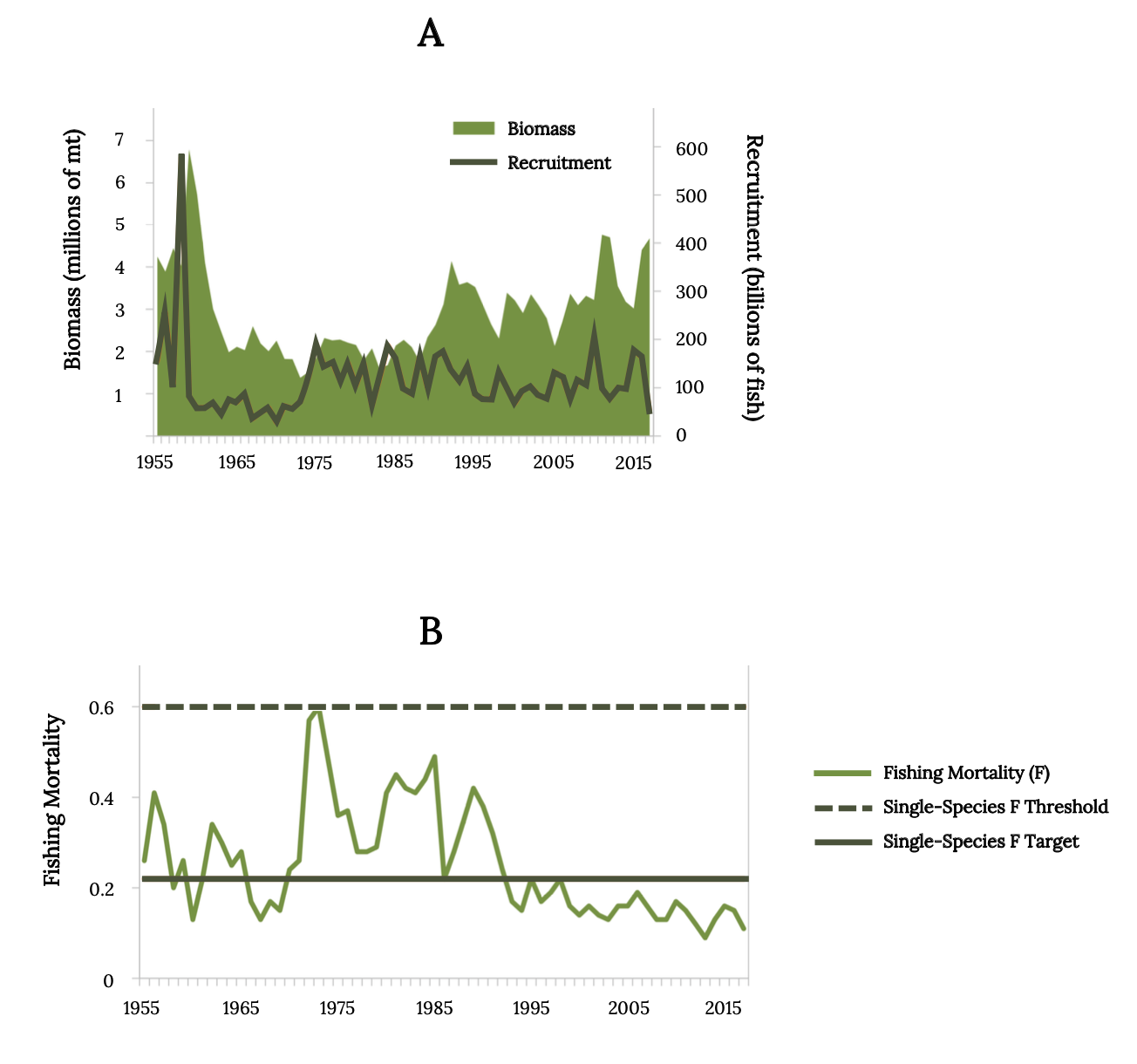
Monitoring records of juvenile Atlantic Menhaden in Chesapeake Bay indicate that reproductive success has been low for many decades (Figure 14.14 top). Fishing mortality for Atlantic Menhaden has been below the single-species management threshold in recent decades (Figure 14.14 bottom). Consequently, the Atlantic Menhaden stock status was not overfished, and overfishing is not occurring (SEDAR 2020a).
Questions to ponder:
What risks are of most concern to you if fishery management continues to make harvest decisions based on single-species analysis? Who is most likely to be harmed by menhaden overharvest?
14.7 Shift from Maximum Sustainable Yield to Ecosystem-Based Management
Fish resources cannot be stored in the sea, they die.
—Chapman 1955, cited in Finley 2011
Early studies of population dynamics of Atlantic Menhaden determined biological reference points, such as maximum sustainable yield, based on a false assumption that they were unaffected by predator abundance and that their natural mortality was a constant. For example, analysts assumed that 36% of Atlantic Menhaden would die each year in the absence of fishing, based on extensive tagging studies during a period when stocks of Striped Bass and other piscivores were at moderate-to-low levels (Ahrenholz et al. 1987). It is hard to imagine or justify that the death rate from predators, diseases, and parasites would be constant over a longer time frame and fixed for all ages. The menhaden story illustrates the scientific principle that Everything Is Connected to Everything Else (the First Law of Ecology) and, therefore, single-species management is ill advised (Pikitch et al. 2012).
By 2000, modern forage fish management recognized that menhaden, herring, and sardine indirectly influenced multiple organisms dependent on forage fish. Management of menhaden had become complicated by many stakeholders concerned with the status of large fish, such as cod, salmon, Striped Bass, sharks, and tuna, as well as seabirds, sea lions, whales, and dolphins that feed on forage fish. Industrial fishing and predators both rely on menhaden, which are more vulnerable to collapse from fishing when predators become more abundant. While early ASMFC management documents acknowledged menhaden’s role as a forage fish, ecological objectives were not added until 2001 (ASMFC 2001). For the next two decades, managers and scientists worked on collecting data and developing models that would assess the species with consideration for the role it plays in the ecosystem. In 2020, the ASMFC (SEDAR 2020) provided ecological reference points to permit the management to protect and maintain the important ecological role Atlantic Menhaden play along the coast (Chagaris et al. 2020; Drew et al. 2021). This was the beginning of a precedent-setting shift from single-species maximum sustainable yield management to ecosystem-based management.
Similar forage fishery management controversies exist worldwide, where forage species such as anchovy, sardine, and other forage species support large industrial fisheries, and needs for supporting fish, mammal, and bird predators is poorly quantified (Pikitch et al. 2012, 2018; Grémillet et al. 2016, 2018; Hilborn et al. 2017). Chesapeake Bay supports a large biomass of age 1 and 2 menhaden as well as age-0 juveniles that recruit to the bay as larvae from ocean spawning. Although Atlantic Menhaden are highly productive, their short life spans mean that sudden changes in population sizes can occur, and the risk of collapse is enhanced by overfishing.
The current demand for Atlantic Menhaden for fish oil and meal is filled largely by one company, Omega Protein, a subsidiary of Cooke Aquaculture. Consequently, the benefits of the fishery are concentrated in the local economy. Reductions in menhaden quotas influence local jobs, county economic outputs, and profits to the company (Kirkley et al. 2011). Quota reductions would reduce local benefits but lead to potential increases in recreational angling, charter boat income, and other jobs. Yet, this menhaden monopoly has not proven to be protection from competition. Just as other products replaced fertilizers and industrial oils produced from menhaden, we can expect the fish oil and fish meal products from menhaden to be replaced by cheaper alternatives. When the need for products produced by menhaden can be met by other products, demand will decrease.
The Atlantic Menhaden at one time ranged from Nova Scotia to Florida. However, immense schools of the fish became less commonplace to many observers. The contraction in the range of Atlantic Menhaden led many environmental groups to become vocal advocates for reducing quotas. In other forage fish, size of fish harvested by the fishery is very similar to the size eaten by seabirds (Pikitch 2012, 2014). In Chesapeake Bay and on the Atlantic Coast, many fish consume Atlantic Menhaden, including Striped Bass (Morone saxatilis), Atlantic Weakfish (Cynoscion regalis), Bluefish (Pomatomus saltatrix), and Bluefin Tuna (Thunnus thynnus). These fish vary in size, so that any fishing on Atlantic Menhaden will likely influence some predators. Models used in previous analysis were frequently inadequate for estimating the impact of fishing forage species on their predators (Pikitch et al. 2017; Hilborn et al. 2017).
Those working to rebuild populations of whales, Bluefin Tuna, Bluefish, Striped Bass, and. Atlantic Weakfish have long challenged the goals of Atlantic Menhaden management. In the 21st century, managers are in the process of transitioning to a new management goal that recognizes that Atlantic Menhaden provide important ecosystem services, including (1) supporting predators as a food resource, (2) supporting a large, directed fishery, and (3) filtering phytoplankton from the water column, mostly as age-0 juveniles.
Incorporating such ecosystem-based goals in management means that quotas will need to be set to provide more forage fish for Striped Bass, Bald Eagles, and other predators. In August 2017, the ASMFC Atlantic Menhaden Management Board approved Draft Amendment 3 to the Fisheries Management Plan. The decision was influenced by a study of the northwest Atlantic ecosystem model, which showed that “birds, highly migratory species, sharks, and marine mammals were . . . negatively affected by increased fishing on menhaden,” though none so much as the Striped Bass (Buchheister et al. 2017a, 2017b). This important scientific finding emphasized that menhaden abundance significantly impacts predator population abundance. Higher fishing mortality on menhaden would mean fewer large menhaden to feed an enhanced population of Striped Bass, as well as reduced abundance of large menhaden during spawning. If Striped Bass were capable of depleting prey populations (Uphoff and Sharov 2018), then they are competing with the menhaden fishery for the very same fish. The draft amendment was the first proposal that considers the use of ecological reference points (ERPs) to manage the resource and changes to the allocation method. In addition, it presents a suite of management options for quota transfers, quota rollovers, incidental catch, the episodic events set aside program, and the Chesapeake Bay reduction fishery cap.
The timeline for key elements in Atlantic Menhaden management are summarized below.
Timeline of Important Management Actions Affecting Atlantic Menhaden
August 2005: First harvest limit on menhaden in Chesapeake Bay imposed by Atlantic States Marine Fisheries Commission (ASMFC)
October 2012: Chesapeake Bay Foundation (CBF) calls for reductions in catch
December 2012: ASMFC adopts a new management plan aimed at reducing harvest
February 2013: Virginia General Assembly passes bill reducing menhaden harvest
March 2014: Virginia Marine Resources Commission creates harvest allocation for bait fishery and reporting requirements for menhaden harvested in Virginia
May 2015: Chesapeake Bay Foundation urges ASMFC to consider ecological reference points in management plan
August 2016: ASMFC delays decision on menhaden harvest cap
October 2016: ASMFC increases the menhaden harvest quota despite lack of data to support an increase
October 2017: A group of more than 100 top ecologists urged the ASMFC to move forward with ecosystem-based management for Atlantic Menhaden
November 2017: ASMFC decreases the cap menhaden harvest and continues to evaluate ecological reference points
February 2018: Coalition of conservation and recreational fishing interests supports new legislation that would ensure Virginia avoids the consequences of falling out of compliance with the menhaden fishery management plan
March 2018: Menhaden legislation is stalled in Virginia General Assembly
August 2018: ASMFC postpones a motion to declare Virginia out of compliance with menhaden plan
December 2018: CBF opposes the certification of Omega Protein’s sustainable menhaden fishery
February 2019: ASMFC commits to further study of ecological effects of menhaden harvest
March 2019: CBF objects to seafood sustainability certification for Omega Protein’s Atlantic menhaden fishery
August 2019: Omega Protein application for sustainability certification challenged
September 2019: Omega Protein knowingly violates the menhaden harvest cap
October 2019: ASMFC finds Virginia out of compliance with harvest cap
November 2019: Virginia governor asks U.S. Secretary of Commerce to impose moratorium on Virginia’s menhaden harvest
December 2019: U.S. Secretary of Commerce supports ASMC, announces deadline for compliance
February 2020: Virginia General Assembly passes legislation to transfer management authority from General Assembly to the Virginia Marine Resources Commission
April 2020: VMRC imposes new menhaden harvest cap to bring Virginia into compliance
August 2020: ASMFC adopts new ecological reference points to guide menhaden management
Source: Chesapeake Bay Foundation. https://www.cbf.org/about-the-bay/more-than-just-the-bay/chesapeake-wildlife/menhaden/timeline-of-menhaden-conservation.html.
The argument that was developed during the period of menhaden controversy can be summarized as follows:
An Argument for Reduced Menhaden Quota
Premises:
- Menhaden are a keystone species; their filter feeding clarifies the water, allowing sunlight to reach eelgrass beds, thereby promoting scallop and juvenile fish habitat.
- Menhaden provide one source of food for Striped Bass, Bluefish, Weakfish, and fluke, as well as whales, all of which are valuable to the recreational economy of the region.
- Products from menhaden can be naturally derived from other sources:
- Chorella and spirulina are high in Omega-3 fatty acids, minerals, and antioxidants.
- Manufacturers are working on canola, which is high in omega-3 fatty acids.
- Marine recreational fishing on sportfish is dependent on menhaden for food and produces high economic benefits and more jobs than commercial fishing.
Claim:
- Quota on menhaden should be reduced to benefit other parts of the ecosystem and the local economy.
The argument for reduced menhaden quotas implies that fisheries management targets for predator and prey cannot be developed in isolation (Drew et al. 2021). Rather, there are tradeoffs in fisheries management due to the simple law that a fish can only die once. A fish harvested by the menhaden reduction fishery cannot also feed Striped Bass. If commercial fleets harvest menhaden at higher rates, there will be lower abundance of predators, such as Striped Bass. Alternatively, reduced fishing mortality for Striped Bass will result in higher predation mortality on menhaden.
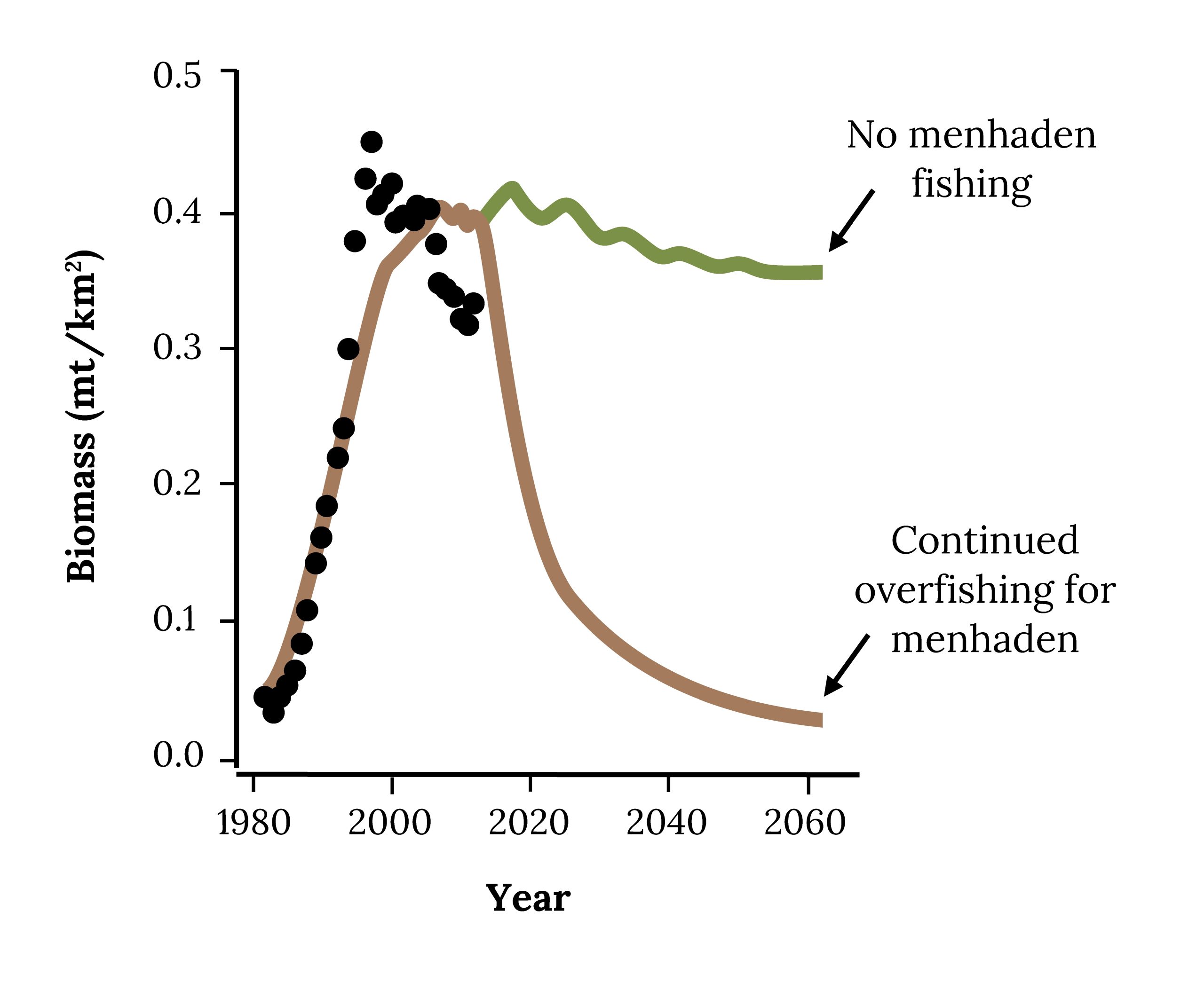
When the reduction industry asks, “Can we harvest more menhaden?” the answer appears to be “Yes.” However, higher fishing on Atlantic Menhaden will likely reduce the biomass of Striped Bass (Figure 14.15) and other high-profile fish that people eat and love to catch, such as Bluefish and Weakfish.
On August 5, 2020, at their meeting in Arlington, the Atlantic States Marine Fisheries Commission voted to implement ecological reference points (ERP) to manage Atlantic Menhaden. ERPs are numeric benchmarks used by managers to promote not only the sustainable harvest of menhaden but also broader ecosystem needs, such as supporting key predators (SEDAR 2020b). Three ecological reference points were adopted in the management of Atlantic Menhaden:
- ERP target: the maximum fishing mortality rate (F) on Atlantic Menhaden that sustains Atlantic Striped Bass at their biomass target when Striped Bass are fished at their F target
- ERP threshold: the maximum F on Atlantic Menhaden that keeps Atlantic Striped Bass at their biomass threshold when Striped Bass are fished at their F target
- ERP fecundity target and threshold: the long-term equilibrium fecundity that results when the population is fished at the ERP F target and threshold, respectively
The adoption of menhaden ecological reference points resulted from a transparent and balanced approach that was informed by science and consistent investments in objective, peer-reviewed research. The menhaden may provide a prime example of ecosystem-based management for other fisheries to strategically plan and implement (Chagaris et al. 2020).
14.8 Stakeholders and Conflicting Values
At a time of precedent-setting change in management, in 2019, the Atlantic Menhaden fishery achieved approval for meeting the Marine Stewardship Council (MSC) certification standards. Fisheries that carry the council’s blue checkmark are required to follow internationally recognized best practices for operating healthy, sustainable fisheries. The MSC standards are considered perhaps the strictest and most reliable, with 28 indicators of seafood sustainability. Atlantic Menhaden fishing with purse seines collects minimal amounts of bycatch, and harvests have been monitored effectively for many decades, thereby permitting estimation of reference points and adjustment of quotas. The MSC fishery standards are based on three core principles that every fishery must meet:
- Sustainable fish stocks: Fishing activity must be at a level that ensures it can continue indefinitely.
- Minimizing environmental impact: Fishing operations must be managed to maintain the structure, productivity, function, and diversity of the ecosystem.
- Effective management: The fishery must comply with relevant laws and have a management system that is responsive to changing circumstances.
However, special interest groups objected to the certification on the grounds that it recognized only the health of the Atlantic Menhaden fishery and not the species’ role in the ecosystem. The Theodore Roosevelt Conservation Partnership paid $6,500 to the MSC to formally contest the certification. In particular, the certification process does not consider the role that Atlantic Menhaden play in supporting Striped Bass, and declines in Striped Bass are a major concern of recreational fishing interests. The fight against MSC certification is a conflict that is best understood in terms of the stories told by stakeholders.
The Atlantic Menhaden conflict is similar to others in which forage fish are harvested in places where valuable sport and commercial fisheries depend on forage fish. The conflict has persisted for decades. As it played out with Atlantic Menhaden, stories told by managers, stakeholders, and scientists each conveyed differing reasons why we needed to account for menhaden’s role as a forage species. However, until recently fisheries management of predators and prey was not well coordinated. Commercial landings of Striped Bass peaked in 1973, and then recreational fishing increased (Richards and Rago 1999). Quotas were changed for Atlantic Menhaden and Striped Bass, but scientists were not able to predict the effects of predator-prey links. The demand for fish oil and fish meal products increased, and menhaden harvesters lobbied for higher quotas. After decades of careful management of harvest for Striped Bass, recovery of their populations influenced predation pressure on Atlantic Menhaden (Uphoff and Sharoff 2018). The recreational Striped Bass anglers had fished before and after the fishing moratorium witnessed changes and told the story of the expected link with menhaden. Vocal activists played a significant role in criticizing Omega Protein’s operations and mobilizing support for reduced quotas, especially in federal waters off New York and New Jersey (e.g., Menhaden Defenders and Theodore Roosevelt Conservation Partnerships). Listening to the many stories that were brought to the Atlantic States Marine Fisheries Commission meetings emphasizes the importance of dealing with conservation conflicts over forage fish as stories to understand and not problems to solve (Harrison and Loring 2020).
The dynamics of the menhaden story will be important to follow in the future, as it is one of the first pelagic, forage fisheries to adopt ecological reference points and at the same time receive sustainability certification. Globally, small pelagics contribute over 15% of the marine fisheries yields, and over three-fourths of that contribution is from developing countries. The future of sustainability certification for menhaden and others will require that management systems are in place to safeguard forage fish in order to protect the stability of top predators from widely fluctuating food levels (Essington et al. 2015; Izquierdo-Peña et al. 2020). The menhaden management story is ongoing, and the future responses will inform managers of the validity of the approach that was adopted in 2020.
Questions to ponder:
What stakeholders in menhaden management are represented? Which stakeholders were not included? What are the stories told by different stakeholders? How do these stories help understand conflict and select appropriate intervention? Can you associate each stakeholder with a preferred management action?
Profile in Fish Conservation: Kristen Anstead, PhD

Kristen Anstead, PhD, is Stock Assessment Scientist with the Atlantic States Marine Fisheries Commission. In this role, she is responsible for periodically analyzing the status of fished populations, including the horseshoe crabs, American Eel, Atlantic Sturgeon, Atlantic Menhaden, and others. In addition to her work for the ASMFC, since 2013 she has been Science Editor and, since 2019, Co-Chief Editor for Fisheries, a leading fisheries science publication.
Dr. Anstead grew up in Maine and attended Bates College, a small, liberal arts college in Lewiston, where she earned a B.S. degree in biology. After graduating from Bates, she worked as a field biologist in several jobs. In the Mpala Research Center, Nanyuki, Kenya, she assisted in an ecology program to improve understanding of the effects of cattle grazing on the diversity and abundance of plants and wild animals. As a field biologist with the University of Georgia Marine Institute, located on Sapelo Island, Georgia, she was able to contribute to the Georgia Coastal Ecosystems Long-Term Ecological Research.
As a Fisheries Observer in Alaska, she worked onboard commercial fishing boats operating in the Bering Sea and Gulf of Alaska. Fisheries observers are the eyes and ears on the water and witness new findings in commercial fisheries. Many fisheries specialists report that their experiences as a fishery observer was a great stepping-stone to a successful fisheries career.
After 10 years of experience as a field biologist, Kristen enrolled in graduate studies at Old Dominion University. She led a novel study to investigate the contribution of multiple nursery areas to the population of Atlantic Menhaden before joining the ASMFC as a Stock Assessment Scientist. Since 2015, her work has contributed to numerous stock assessments conducted by the Atlantic States Marine Fisheries Commission. Her knowledge, skills, and abilities from her years as a field biologist, along with her specialties, mean that she brings a unique balance to her work in stock assessment. As a Stock Assessment Scientist for Atlantic Menhaden, she is frequently reminded how much people care about menhaden, a noncharismatic fish that people will never see on a restaurant menu.
Dr. Anstead encourages students to pursue fieldwork positions to get some hands-on experience and an understanding of how science interacts with communities. For students interested in the management process, everything produced by the ASMFC is in the public domain to ensure that decisions are made in the public interest. See more about the Atlantic States Marine Fisheries Commission here.
Key Takeaways
- Menhaden sustained a large and important fishery for Native Americans and later early European colonists.
- Landings of menhaden fisheries are the largest by volume on the Atlantic Coast.
- Menhaden fishery supports jobs, and menhaden are transformed to useful products, most importantly fish oils and meals.
- Life history of Atlantic Menhaden represents an opportunistic strategy characterized by many small offspring, fast growth, early maturity, and small adult body size.
- Menhaden are grouped with other fish that eat plankton and are eaten by predatory fish, squid, birds, and mammals.
- Menhaden and other small, pelagic forage fish are highly responsive to climate variation.
- High levels of fishing effort increase the risk of collapse of menhaden.
- Management of Atlantic Menhaden recently adopted ecological reference points in order to adjust quotas in response to abundance of predators, such as Striped Bass.
This chapter was reviewed by Kristen Anstead.
URLs
Sharks entering a large school of menhaden: https://www.nationalgeographic.com/news/2017/08/shark-feeding-frenzy-menhaden-school-hamptons-drone-video-spd
Draft Amendment 3: http://www.asmfc.org/files/PublicInput/%20AtlanticMenhadenDraftAmendment3_%20PublicComment.pdf
Atlantic States Marine Fisheries Commission: http://www.asmfc.org/
Long Descriptions
Figure 14.3: Illustration of purse seine: large wall of netting deployed around an entire area or school of fish. The seine has floats along the top line with a lead line threaded through rings along the bottom. Jump back to Figure 14.3.
Figure 14.4: Line graph with x-axis as fork length (cm) from 0-40 and y-axis as fecundity (thousands) from 0-500. Line show exponential curve with lowest point at 15 cm/0 fecundity, increasing to 35cm/500 fecundity. Jump back to Figure 14.4.
Figure 14.10: Landings of Atlantic Menhaden over time; 1) 1955-1980, boom, bust, and recovery; 2) 1980-2000, beginning of coastwide management; 3) 2000-2010, first steps toward ecosystem modeling; 4) 2010-2015, first coastwide quota and lenfest report; 5) 2015- ;amendment 3 and the ERP assessment. Jump back to Figure 14.10.
Figure 14.11: Population biomass increases as yield decreases. MSY shows parabolic relationship between equilibrium yield, population biomass, and intrinsic rate of population increase. Jump back to Figure 14.11.
Figure 14.12: Two scatter plots with fitted lines. A: As effort increases, catch per unit of effort decreases. B: Parabolic shape opening downward shows highest point at effort=1000, catch=600. Jump back to Figure 14.12.
Figure 14.14: Top graph shows Atlantic Menhaden biomass and recruitment from 1955 to 2016. Biomass and recruitment peak in 1955. Bottom graph shows atlantic menhaden fishing mortality from 1955 to 2016. Single species F thresholh remains at 0.6 mortality; single species F target remains at 0.2 mortality. Fishing mortality peaks at 1971. Jump back to Figure 14.14.
Figure 14.15: Biomass has continually increased from 1980-2020. If overfishing continues for menhaden, the graph will begin to trend downwards. If there is no menhaden fishing, graph will level out. Jump back to Figure 14.15.
Figure References
Figure 14.1: Illustration of the Atlantic Menhaden adult. Artemas Ward, 1923. Public domain. https://commons.wikimedia.org/wiki/File:Menhaden,_photo_from_The_Encyclopedia_of_Food_by_Artemas_Ward.jpg.
Figure 14.2: Map of the range of Atlantic Menhaden from Nova Scotia, Canada, along the nearshore and coastal waters of the United States Atlantic Coast to Florida. NOAA via The Path to an Ecosystem Approach for Forage Fish Management: A Case Study of Atlantic Menhaden, by Anstead et. al., 2021. CC BY 4.0. http://dx.doi.org/10.3389/fmars.2021.607657.
Figure 14.3: Purse seines were adopted early on as a preferred method for harvesting menhaden. Benjamin Franklin Conklin, 1887. Public domain. https://commons.wikimedia.org/wiki/File:FMIB_45912_Menhaden_Fishery.jpeg.
Figure 14.4: Relationship between fork length (cm) and predicted number of ova (fecundity) for Atlantic Menhaden. Kindred Grey. 2022. CC BY 4.0. Data from Fecundity of Atlantic Menhaden, Brevoortia tyrannus, by Lewis et. al., 1987. https://doi.org/10.2307/1351894.
Figure 14.5: Illustration of the (A) first gill arch showing gill rakers (a) and gill lamellae (m); and (B) enlarged section of six gill rakers showing fine rows of hooks for filter feeding. Hugh McCormick Smith, 1907. Public domain. https://commons.wikimedia.org/wiki/File:FMIB_51375_Gill_of_Mehnaden.jpeg.
Figure 14.6: Simplified food web showing the links that Atlantic Menhaden provide between plankton and carnivorous animals. Kindred Grey. 2022. CC BY 4.0. Includes Brevoortia Patronus, by SEFSC Pascagoula Laboratory; Collection of Brandi Noble, NOAA/NMFS/SEFSC, 2008 (CC BY 2.0, https://commons.wikimedia.org/wiki/File:BrevoortiaPatronus.jpg), Bluefin-big, by NOAA, 2004 (public domain, https://commons.wikimedia.org/wiki/File:Bluefin-big.jpg), fishing ship, by Gan Khoon Lay, 2019 (Noun Project license, https://thenounproject.com/icon/fishing-ship-2760125/), seagull, by Daniela Baptista, 2016 (Noun Project license, https://thenounproject.com/icon/seagull-781412/), Humpback Whale, by Philipp Lehmann, 2017 (Noun Project license, https://thenounproject.com/icon/humpback-whale-957308/), and bass fish, by Phạm Thanh Lộc, 2019 (Noun Project license, https://thenounproject.com/icon/bass-fish-3385868/).
Figure 14.7: Osprey in flight with a menhaden held in its talons. Russ, 2016. CC BY 2.0. https://flic.kr/p/KV7Yym.
Figure 14.8: Relationship between the size of Striped Bass (total length, mm) and the size of prey fish (total length, mm) consumed. Kindred Grey. 2022. CC BY 4.0. Data from Interactions between Adult Migratory Striped Bass (Morone saxatilis) and Their Prey during Winter off the Virginia and North Carolina Atlantic Coast from 1994 through 2007, by Overton et. al. 2008. http://hdl.handle.net/1834/19906.
Figure 14.9: Purse-seine boats encircling a school of menhaden. Robert K. Brigham, 1968. Public domain. https://commons.wikimedia.org/wiki/File:Menhaden_fishing_-_purse_seine_boats.jpg.
Figure 14.10: Atlantic Menhaden landings (thousands of metric tons, mt) from the reduction and bait fisheries during each of the five periods of assessment and management history. Coastwide harvest quotas began in 2013 and are indicated on the graph in red. Kindred Grey. 2022. CC BY 4.0. Data from The Path to an Ecosystem Approach for Forage Fish Management: A Case Study of Atlantic Menhaden, by Anstead et. al. 2021. https://doi.org/10.3389/fmars.2021.607657.
Figure 14.11: Relationship between equilibrium yield (Y, green curve) and intrinsic rate of population increase (r, tan line) and population biomass with the maximum sustainable yield (dashed line). Kindred Grey. 2022. CC BY 4.0. Data from Maximum Sustainable Yield, by Tsikliras and Froese, 2018. https://doi.org/10.1016/B978-0-12-409548-9.10601-3.
Figure 14.12: (A) Straight line fitted to fisheries data on catch per unit effort and effort (vessel-weeks) for Atlantic Menhaden from 1955 to 1969. (B) Total catch plotted against effort. Kindred Grey. 2022. CC BY 4.0. Data from Effects of Fishing on the Atlantic Menhaden Stock: 1955–1969, by W. E. Schaaf and G. R. Huntsman, 1972. https://doi.org/10.1577/1548-8659(1972)101<290:EOFOTA>2.0.CO;2.
Figure 14.13: Annual mortality against effort for 1955–1966. Kindred Grey. 2022. CC BY 4.0. Data from Effects of Fishing on the Atlantic Menhaden Stock: 1955–1969, by W. E. Schaaf and G. R. Huntsman, 1972. https://doi.org/10.1577/1548-8659(1972)101<290:EOFOTA>2.0.CO;2.
Figure 14.14: (A) Estimated Atlantic Menhaden biomass and recruitment from 1955 to 2016. (B) Atlantic Menhaden fishing mortality (ages 2–4) from 1955 to 2016 with lines depicting management target (solid) and threshold (dashed). Kindred Grey. 2022. CC BY 4.0. Data from Maximum Sustainable Yield, by Tsikliras and Froese, 2018. https://doi.org/10.1016/B978-0-12-409548-9.10601-3.
Figure 14.15: Projected biomass of Striped Bass in future under different fishing mortality rates for Atlantic Menhaden. Kindred Grey. 2022. CC BY 4.0. Adapted from Evaluating Ecosystem-Based Reference Points for Atlantic Menhaden (Brevoortia tyrannus), by Buchheister et. al., 2017. CC BY 4.0. http://dx.doi.org/10.1080/19425120.2017.1360420.
Figure 14.16: Kristen Anstead, PhD. Used with permission from Kristen Anstead. CC BY 4.0.
Text References
Ahrenholz, D. W., W. R. Nelson, and S. P. Epperly. 1987. Population and fishery characteristics of Atlantic Menhaden, Brevoortia tyrannus. United States Fishery Bulletin 85(3):569–600.
Anstead, K., K. Drew, D. Chagaris, M. Cieri, A. M. Schueller, J. E. McNamee, A. Buchheister, G. Nesslage, J. H. Uphoff Jr., M. J. Wilberg, A. Sharov, M. J. Dean, J. Brust, M. Celestino, S. Madsen, S. Murray, M. Appelman, J. C. Ballenger, J. Brito, E. Cosby, C. Craig, C. Flora, K. Gottschall, R. J. Latour, E. Leonard, R. Mroch, J. Newhard, D. Orner, C. Swanson, J. Tinsman, E. D. Houde, T. J. Miller, and H. Townsend. 2021. The path to an ecosystem approach for forage fish management: a case study of Atlantic Menhaden. Frontiers in Marine Science 8. https://doi.org/10.3389/fmars.2021.607657.
Arellanes, I. C., N. Choe, V. Solomon, X. He, B. Kavin, A. E. Martinez, N., Kono, D. P. Buennagel, N., Hazra, G., Kim, and L. M. D’Orazio. 2020. Brain delivery of supplemental docosahexaenoic acid (DHA): a randomized placebo-controlled clinical trial. EBioMedicine 59(2):102883. DOI: https://doi.org/10.1016/j.ebiom.2020.102883.
ASMFC. 2001. Amendment 1 to the Interstate Fishery Management Plan for Atlantic Menhaden. Fishery Management Report No. 37, Atlantic States Marine Fisheries Commission. Available at: https://www.asmfc.org/uploads/file/menhadenAm_1.PDF.
ASMFC. 2020. ASMFC Atlantic Menhaden Board prepares to move forward with Menhaden ecological reference points. News Release, Atlantic States Marine Fisheries Commission. February 7.
ASMFC. 1999. Atlantic Menhaden stock assessment report for peer review. Stock Assessment Report No. 99-01, Atlantic States Marine Fisheries Commission. Available at: http://www.asmfc.org/uploads/file/feb99menhadenPeerReviewrpt.pdf.
ASMFC. 1981. Fishery Management Plan for Atlantic Menhaden. Fishery Management Report No. 2, Atlantic States Marine Fisheries Commission. Available at: http://www.asmfc.org/uploads/file/1981MenhadenFMP.pdf.
Brown, D. M., J. Robbins, P. L. Sieswerda, C. Ackerman, J. M. Aschettino, S. Barco, T. Boye, T., R. A. DiGiovanni, K. Durham, A. Engelhaupt, and A. Hill. 2022. Site fidelity, population identity and demographic characteristics of Humpback Whales in the New York Bight apex. Journal of the Marine Biological Association of the United Kingdom 102(1-2):157–165.
Brown, D. M., J. Robbins, P. L. Sieswerda, R. Schoelkopf, and E. C. M. Parsons. 2018. Humpback Whale (Megaptera novaeangliae) sightings in the New York–New Jersey Harbor estuary. Marine Mammal Science 34:250–257.
Buchheister, A., T. J. Miller, and E. D. Houde. 2017a. Evaluating ecosystem-based reference points for Atlantic Menhaden. Marine and Coastal Fisheries 9:457–478.
Buchheister, A., T. J. Miller, E. D. Houde, and D. A. Loewensteiner. 2017b. Technical documentation of the northwest Atlantic continental shelf (NWACS) ecosystem model. Report to the Lenfest Ocean Program, Washington, D.C. University of Maryland Center for Environmental Sciences Report TS-694-17. Available at: http://hjort.cbl.umces.edu/NWACS/TS_694_17_NWACS_Model_Documentation.pdf.
Butler, C. M., P. J. Rudershausen, and J. A. Buckel. 2010. Feeding ecology of Atlantic Bluefin Tuna (Thunnus thynnus) in North Carolina: diet, daily ration, and consumption of Atlantic Menhaden (Brevoortia tyrannus). U.S. National Marine Fisheries Service Fishery Bulletin 108:56–69.
Chagaris, D., K. Drew, A. Schueller, M. Cieri, J. Brito, and A. Buchheister. 2020. Ecological reference points for Atlantic Menhaden established using an ecosystem model of intermediate complexity. Frontiers in Marine Science 7:606417. https://doi.org/10.3389/fmars.2020.606417.
Checkley, D. M., R. G. Asch, and R. R. Rykaczewski. 2017. Climate, anchovy, and sardine. Annual Review of Marine Science 9:469–493.
Deyle, E., A. M. Schueller, H. Ye, G. M. Pao, and G. Sugihara. 2018. Ecosystems-based forecasts of recruitment in two menhaden species. Fish and Fisheries 19:769–781.
Drew, K., M. Cleri, A. M. Schueller, A. Buchheister, D. Chagaris, G. Nesslage, J. E. McNamee, and J. H. Uphoff Jr. 2021. Balancing model complexity, data requirements, and management objectives in developing ecological reference points for Atlantic Menhaden. Frontiers in Marine Science 8:608059. https://doi.org/10.3389/fmars.2021.608059.
Essington, T. E., P. E. Moriarty, H. E. Froehlich, E. E. Hodgson, L. E. Koehn, K. L. Oken, M. C. Siple, and C. C. Stawitz. 2015. Fishing amplifies forage fish population collapses. Proceedings of the National Academy of Sciences of the United States of America 112(21):6648–6652.
Finley, C. 2011. All the fish in the sea: maximum sustainable yield and the failure of fisheries management. University of Chicago Press. DOI: 10.7208/chicago/9780226249681.001.0001.
Franklin, H. B. 2008. The most important fish in the sea: menhaden and America. Shearwater Press, Washington, D.C.
Glass, K. A., and B. D. Watts. 2009. Osprey diet composition and quality in the high- and low-salinity areas of lower Chesapeake Bay. Journal of Raptor Research 43:27–36.
Goode, G. B. 1887. Fisheries and fishery industries of the United States. Government Printing Office, Washington, D.C.
Goode, G. B. 1880. A history of the menhaden. Orange Judd, New York. Available at: https://www.biodiversitylibrary.org/item/40759.
Gordon, C. A., D. A. Cristol, and R. A. Beck. 2000. Low reproductive success of Black Skimmers associated with low food availability. Waterbirds: The International Journal of Waterbird Biology 23:468–474.
Harrison, H. L., and P. A. Loring. 2020. Seeing beneath disputes: a transdisciplinary framework for complex conservation conflicts. Biological Conservation 248:108670. https://doi.org/10.1016/j.biocon.2020.108670.
Hilborn, R., R. O. Amorosa, E. Bogazzi, O. P. Jensen, A. M. Parma, C. Szuwalski, and C. J. Walters. 2017. When does fishing forage species affect their predators? Fisheries Research 191:211–221.
Hong, M. Y., J. Lumibao, P. Mistry, R. Saleh, and E. Hoh. 2015. Fish oil contaminated with persistent organic pollutants reduces antioxidant capacity and induces oxidative stress without affecting its capacity to lower lipid concentrations and systemic inflammation in rats. Journal of Nutrition 145:939–944. doi: 10.3945/jn.114.206607.
Izquierdo-Peña, V., S. E. Lluch-Cota, F. P. Chavez, D. B. Lluch-Cota, E. Morales-Bojórquez, and G. Ponce-Díaz. 2020. Is there a future in the sustainability certification of sardine and anchovy fisheries? Fisheries 45(10):554–560.
Jensen, A. L. 1975. Computer simulation of effects on Atlantic Menhaden yield of changes in growth, mortality, and reproduction. Chesapeake Science 16:139–142.
Jensen, A. L. 1976. Time series analysis and forecasting of Atlantic Menhaden catch. Chesapeake Science 17:305–307.
Kirkley, J. E., T. Hartman, T. McDaniel, K. McConnell, and J. Whitehead. 2011. An assessment of the social and economic importance of Menhaden (Brevoortia tyrannus) (Latrobe, 1802) in Chesapeake Bay region. VIMS Marine Resource Report No. 2011-14, Gloucester Point, VA. Available at: https://mrc.virginia.gov/vsrfdf/pdf/RF09-11_Aug11.pdf. Accessed 18 April 2021.
Lewis, R. M, D. W. Arenholtz, and S. P. Epperly. 1987. Fecundity of Atlantic Menhaden, Brevoortia tyrannus. Estuaries 10:347–350. Available at: https://link.springer.com/content/pdf/10.2307/1351894.pdf.
Liljestrand, E. M., M. J. Wilberg, and A. M. Schueller. 2019. Estimation of movement and mortality of Atlantic Menhaden during 1966–1969 using a Bayesian multi-state mark-recovery model. Fisheries Research 210:204–213.
Lucca, B. M., and J. D. Warren. 2019. Fishery-independent observations of Atlantic Menhaden abundance in the coastal waters south of New York. Fisheries Research 218:229–236.
MacCall, A. D., W.J . Sydeman, P. C. Davison, and J. A. Thayer. 2016. Recent collapse of northern anchovy biomass off California. Fisheries Research 175:87–94.
Merino, G., M. Barange, and C. Mullon. 2010. Climate variability and change scenarios for a marine commodity: modelling small pelagic fish, fisheries and fishmeal in a globalized market. Journal of Marine Systems 81:196–205. https://doi.org/10.1016/j.jmarsys.2009.12.010.
Midway, S. R., A. M. Schueller, R. T. Leaf, G. M. Nesslage, and R. M. Mroch III. 2020. Macroscale drivers of Atlantic and Gulf Menhaden growth. Fisheries Oceanography 29(3):252–264.
National Marine Fisheries Service. 2020. Fisheries of the United States, 2018. U.S. Department of Commerce, NOAA Current Fishery Statistics No. 2018 Available at: https://media.fisheries.noaa.gov/dam-migration/fus_2018_factsheet.pdf
Overton, A. S., J. C. Griffin, F. J. Margraf, E. B. May, and K. J. Hartman. 2015. Chronicling long-term predator responses to a shifting forage base in Chesapeake Bay: an energetics approach. Transactions of the American Fisheries Society 144:956–966.
Overton, A. S., C. S. Manooch III, J. W. Smith, and K. Brennan. 2008. Interactions between adult migratory Striped Bass (Morone saxatilis) and their prey during winter off the Virginia and North Carolina Atlantic Coast from 1994 through 2007. U.S. National Marine Fisheries Service Fishery Bulletin 106(2):174–182.
Peck, J. I. 1894. On the food of the menhaden. Bulletin of the U.S. Fisheries Commission 13:113–126.
Pikitch, E. K., P. D. Boersma, I. L. Boyd, D. O. Conover, P. M. Cury, T. E. Essington, and S. S. Heppell. 2012. Little fish, big impact: managing a crucial link in ocean food webs. Lenfest Ocean Program, Washington, D.C. Available at: https://www.lenfestocean.org/~/media/legacy/lenfest/pdfs/littlefishbigimpact_revised_12june12.pdf?la=en.
Pikitch, E. K., P. D. Boersma, I. L. Boyd, D. O. Conover, P. M. Cury, T. E. Essington, S. S. Heppell, E. D. Houde, M. Mangel, D. Pauly, É. Plagányi, K. Sainsbury, and R. S. Steneck. 2018. The strong connection between forage fish and their predators: a response to Hilborn et al. (2017). Fisheries Research 198. DOI: 10.1016/j.fishres.2017.07.022.
Pikitch, E. K., K. J. Rountos, T. E. Essington, C. Santora, D. Pauly, R. Watson, U. R. Sumaila, P. D. Boersma, I. L. Boyd, D. O. Conover, P. Cury, S. S. Heppell, E. D. Houde, M. Mangel, É. Plagányi, K. Sainsbury, R. S. Steneck, T. M. Geers, N. Gownaris, and S. B. Munch. 2014. The global contribution of forage fish to marine fisheries and ecosystems. Fish and Fisheries 15(1):43–64. doi:10.1111/faf.12004.
Richards, R. A., and P. J. Rago. 1999. A case history of effective fishery management: Chesapeake Bay Striped Bass. North American Journal of Fisheries Management 19:356–375.
Schaff, W. E., and G. R. Huntsman. 1972. Effects of fishing on the Atlantic Menhaden stock: 1955–1969. Transactions of the American Fisheries Society 101:290–297.
Schueller, A. M., and E. H. Williams. 2017. Density-dependent growth of Atlantic Menhaden: impacts on current management. North American Journal of Fisheries Management 37:294–301.
SEDAR. 2020a. SEDAR 69: Atlantic Menhaden benchmark stock assessment report. SouthEast Data Assessment and Review, North Charleston, SC. Available at: http://sedarweb.org/sedar-69.
SEDAR. 2020b. SEDAR 69: Atlantic Menhaden ecological reference points stock assessment report. SouthEast Data Assessment and Review, North Charleston, SC. Available at: http://sedarweb.org/sedar-69.
Sherratt, S. C. R., M. Lero, and R. P. Mason. 2020. Are dietary fish oil supplements appropriate for dyslipidemia management? A review of the evidence. Current Opinion in Lipidology 31(2):94–100. doi: 10.1097/MOL.0000000000000665.
Smith, H. M. 1907. Fishes of North Carolina. North Carolina Geological and Economic Survey, vol. 2. E. M. Uzzell, Raleigh, NC.
Smith, J. W. 1999. Distribution of Atlantic Menhaden, Brevoortia tryrannus, purse-seine sets and catches from southern New England to North Carolina, 1985–96. U.S. Department of Commerce, NOAA Technical Report NMFS 144.
Spitzer, P. R., and A. F. Poole. 1980. Coastal Ospreys between New York City and Boston: a decade of reproductive recovery 1969–1979. American Birds 34:233–242.
Stevick, P. T., J. Allen, P. J. Clapham, N. Friday, S. K. Katona, F. Larsen, J. Lien, D. K. Mattila, P. J. Palsbøll, J. Sigurjónsson, T. D. Smith, N. Øien, and P. S. Hammond. 2003. North Atlantic Humpback Whale abundance and rate of increase four decades after protection from whaling. Marine Ecology Progress Series 258:263–273.
Szuwalski, C. S., and J. T. Thorson. 2017. Global fishery dynamics are poorly predicted by classical models. Fish and Fisheries 18:1085–1095.
Uphoff, J. H. Jr. 2003. Predator-prey analysis of Striped Bass and Atlantic Menhaden in upper Chesapeake Bay. Fisheries Management and Ecology 10:313–322.
Walter, J. F. III, A. S. Overton, K. H. Ferry, and M. E. Mather. 2003. Atlantic Coast feeding habits of Striped Bass: a synthesis supporting a coast-wide understanding of trophic biology. Fisheries Management and Ecology 10:349–360.
World Bank. 2013. Fish to 2030: prospects for fisheries and aquaculture. World Bank Report Number 83177-GLB. Available at: http://www.fao.org/3/i3640e/i3640e.pdf.
Ground dried fish used as fertilizer or animal feed.
Not known in advance or precisely fixed in extent
Ability to produce an abundance of offspring, new growth, or number of eggs
Tidal mouth of a large river, where the tide meets the stream
Those fish that occur in same calendar year
Increase in a natural population as progeny grow and immigrants arrive
Inhabiting the upper layers of a water body
Large group of pelagic fish including herring, shad, menhadens, sardine, anchovy, and their relatives
Feeding on fish
Class of organic compounds that are fatty acids or their derivatives and are insoluble in water but soluble in organic solvents
Main constituent of natural fats and oils, and high concentrations in the blood indicate an elevated risk of stroke
Bright red blood present in most arteries that has been oxygenated in lungs or gills
Substance that removes potentially damaging oxidizing agents in a living organism
Plant grown for its fiber, from which linen is made, and for its seed, from which oil and livestock feed are obtained
Catch that could be taken every year by a fixed amount of fishing effort, maintaining the stock at a constant level, assuming a steady-state situation "at equilibrium" with the total fishing effort in the long term

