13 Grouper and Spawning Aggregations
Learning Objectives
- Describe the life history, characteristics, habitats, and behaviors of grouper that influence their vulnerability to overharvest.
- Define the many roles of grouper in the ecosystem.
- Recognize how conspicuous consumption patterns contribute to overfishing in grouper.
- Describe movements of different stages in the grouper life cycle.
- Suggest appropriate management strategies to restore overfished grouper populations.
13.1 The Grouper: Their Remarkable Life History and Behavior
Grouper are a diverse group of marine fish, which are characterized by their large size and relatively low reproductive rates. The common name, grouper, applies to 175 fish species in the family Epinephelidae, formerly tribe Epinephelini under subfamily Epinephelinae and family Serranidae (Sadovy de Mitcheson and Liu 2022). In other parts of the world, grouper are variously called cabrillas, garropas, gropers, lapu-lapu, pugapo, hapuku, or hammour. The name “grouper” is believed to derive from the Portuguese garoupa. There are sixteen genera of grouper, the most diverse being Epinephelus with 87 species and Mycteroperca with 15 species. Some smaller species of grouper are classified in several other genera, such as Alphestes, Cephalopholis, Cromileptes, Dermatolepis, and Variola.
One allure of the grouper is the massive size reached by some species. The taxon includes the largest of all reef fish (among teleosts), the Giant (Epinephelus lanceolatus), the Pacific Goliath (E. quinquefasciatus), and the Atlantic Goliath (E. itajara) Grouper that can exceed 2 m in total length, although few exceed 1 m (Craig et al. 2011). The Giant Grouper grows up to 2.7 m (8.9 ft) in length and 400 kg (880 lbs) in weight.
The morphologies of grouper are similar in that they typically have a stout body, large head, and large mouth with impressive suction volume. The body form allows them to act as rover predators or ambush predators, usually swallowing a single large prey whole.
Many, but not all, grouper are protogynous hermaphrodites, which means that they first mature and reproduce as females and then transition to males later. Consequently, the sex ratio is typically skewed in favor of females, especially in exploited populations. Large males maintain territories on the coral reefs, rocky outcroppings, or artificial reefs, while females may remain at shallower depths before migrating to these sites during the spawning season. Juvenile grouper typically have a different color pattern and occupy different habitats than adults. They have an episodic life history strategy, with many small offspring, slow growth, late reproduction, large size, and long life spans (Kindsvater et al. 2017; Figure 13.1).
One of the common behaviors of grouper is the formation of large spawning aggregations that occur at consistent locations at specific times of year, times of day, and phases of the moon. Spawning aggregations serve to synchronize spawning time and maximize fertilization success. Elaborate courtship behaviors have been observed during spawning (Erisman et al. 2007), which often occurs near sunset, presumably to minimize mortality of the pelagic eggs from visual predators. Grouper spawning aggregations are also a strong draw for SCUBA divers in many popular tourist destinations, including Palau, Belize, and French Polynesia. Despite the many ecological, social, and economic benefits provided by the grouper, there is often little government interest in management and documenting landings and values in the many small island states. Furthermore, there have been too few studies on effects of pollutants, habitat degradation, and climate change on grouper populations.
13.2 Grouper Habitats

Grouper typically occupy coral and rocky reefs found predominantly in tropical and subtropical areas of the Atlantic and Indo-Pacific regions (Craig et al. 2011; Sadovy de Mitcheson and Liu 2022). Most occur in relatively shallow coastal waters where they are easily fished by locals familiar with the reef structure, but some species extend farther offshore on deeper reefs down to about 300 meters. Like many coral reef fish, adult and juvenile grouper often use very different habitats that are threatened from human modification (Sambrook et al. 2019). Managers must protect the connected, interacting collections of juxtaposed habitat patches to preserve the life cycle of grouper (Mumby 2006). It’s a truism in fish conservation that to conserve fish species, we must conserve their habitat. However, the reality of habitat conservation is for more complex because habitats are dynamic and vary in space and time.
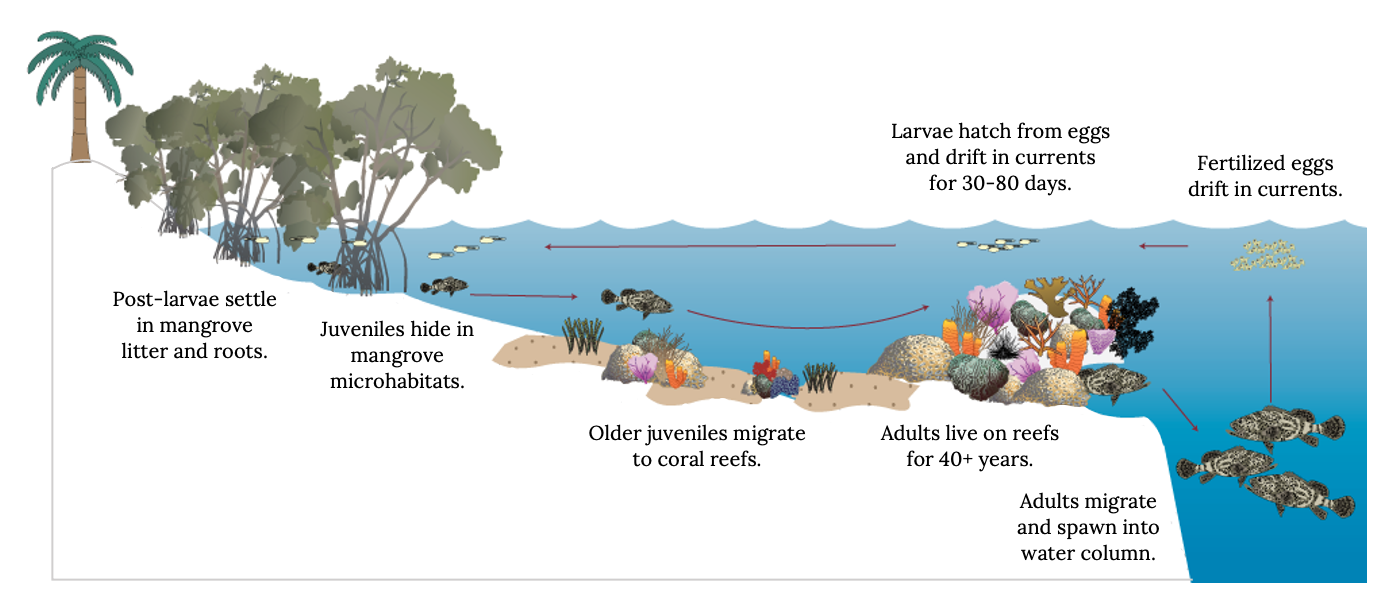
The Atlantic Goliath Grouper is a case in point (Figure 13.2). Their eggs are pelagic, and developing embryos are transported via currents to shallow-water habitats, such as mangroves and seagrass meadows, where they first settle in mangrove leaf litter (Lara et al. 2009). The juvenile habitats are essential for growth and survival to maintain steady recruitment of new adults to the coral reefs. However, these shallow-water habitats are often degraded or transformed to less-productive habitats (Valiela et al. 2001; Aronson et al. 2003; Coté et al. 2005; Waycott et al. 2009; McKenzie et al. 2020). Some grouper make long migrations between nursery habitats and reefs (McMahon et al. 2012). Coral reefs throughout the world are changing due to overfishing, climate change, water quality, ocean acidification, and coral diseases and bleaching (Arundsen et al. 2003). Any declines in coral reef fish or invertebrates directly limit the food base for adult grouper (Russ et al. 2021). Consequently, the recovery of overfished populations, such as the Goliath Grouper populations in Florida, depends on availability of high-quality mangrove habitat in southwest Florida as well as controls on harvest (Koenig et al. 2007; Shideler et al. 2015b).
13.3 Spawning Aggregations and Implications for Fishing
If slow life history and high value create a double jeopardy for grouper, one additional trait adds a triple jeopardy condition. Grouper display spawning aggregations, temporary gatherings of large numbers of grouper for spawning at specific times and places. Location and timing are known by local fishers. In some species, aggregations may be transient—that is, made up of fish that travel long distances and persist for only days or weeks. Others are resident spawning aggregations that involve fish that travel short distances and persist for minutes or hours. These resident aggregations are often timed during the winter full moon (Colin 1992). Male grouper typically arrive at the site first and spend longer than females. Strong spawning-site fidelity is displayed by grouper. One individual returned to the very same spawning site for eight consecutive years (Washckewithz and Wirtz 1990).
These spawning aggregations make grouper extremely vulnerable at the same time that reproductive values are highest (Erisman et al. 2017). As grouper move around, local fishers learn their patterns and can use GPS (global positioning systems) to relocate these spots and target the spawning aggregations. Fisher knowledge influences the extent to which aggregations are perceived as predictable (Robinson et al. 2015). In some cases, fishers have known for centuries where and when aggregations form (Erisman et al. 2017). More of the grouper population can be harvested when fish aggregate to spawn. It’s a phenomenon that fisheries professionals have named hyperstability (Erisman et al. 2011). Because fishermen can’t catch them fast enough, the catch per unit effort remains high even as populations plummet. This results in faulty information on the abundance of grouper stocks (Robinson et al. 2015). Heavy selective fishing pressure on grouper aggregations removes mature older individuals (Coleman et al. 1996). In the case of the Nassau Grouper (Epinephelus striatus), declines were first noticed when spawners failed to show at historical spawning aggregation sites (Coleman et al. 1996; Aguilar-Perera 2006; Aguilar-Perera et al. 2014).
Therefore, effective management requires understanding and consideration of life history and ecological and socioeconomic drivers, as well as strong enforcement of fishing regulations. Active spawning aggregations, due to their discrete nature and high productivity, are clearly important source areas for grouper populations. Hence, these isolated sites support abundance of grouper and represent focal points for establishing no-kill marine reserves (Sadovy and Domeier 2005; Sadovy de Mitcheson 2016; Paxton et al. 2021).
13.4 Grouper and Ecosystem Services
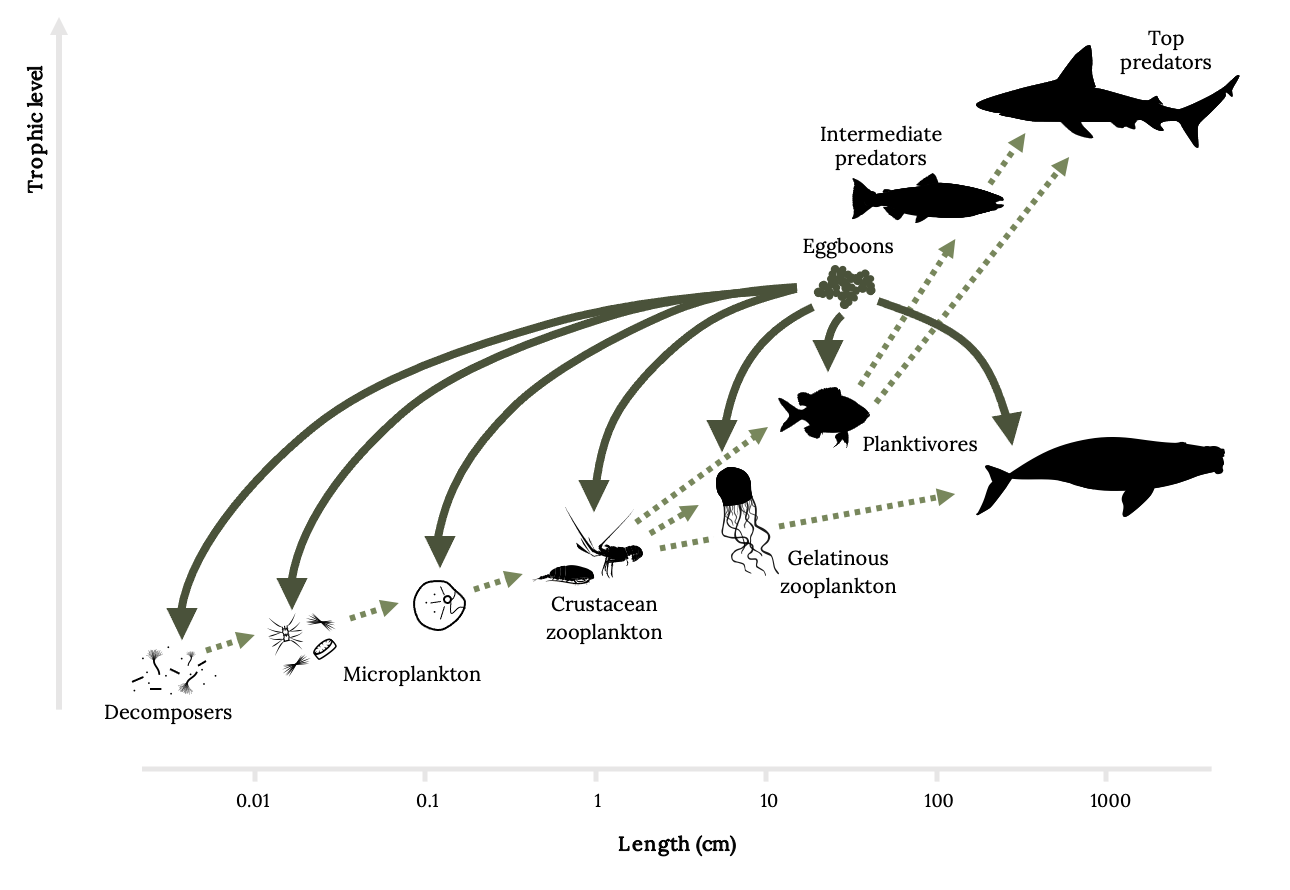
Grouper provide many direct and indirect services in coral reef ecosystems. Spawning aggregations have indirect effects on marine ecosystems. Egg boons are the large, though temporary, egg concentrations that provide highly nutritious fatty acids and support multiple trophic levels (Figure 13.3; Fuiman et al. 2015). Whale sharks also aggregate seasonally to feed on eggs from fish spawning aggregations, attracting tourism that depends on conservation and provides economic returns far more valuable than the capture fisheries (Colman 1997; Sala et al. 2001; Heyman et al. 2001, 2010). Loss of grouper translates to a loss of trophic redistribution via egg boons.
Grouper are among the largest apex predators on coral reefs and are critical for balancing the abundance of many other fish (Hensel et al. 2019), typically damselfish (Pomacentridae) and wrasses (Labridae). Grouper predation may provide some level of biocontrol for invasive lionfish (Maljkovic et al. 2008; Mumby et al. 2011). Spawning aggregations also support high local abundance of sharks (Mourier et al. 2016). Some grouper species, such as the Red Grouper (Epinephelus morio), create habitat structure by clearing away sediment, thereby creating refuges for other fish and invertebrates from predation in these complex habitats (Coleman et al. 2011).
As large predators, grouper contribute to overall high fish abundance, especially on complex reefs (Hensel et al. 2019). Removal of predators from coral reefs releases many invertebrates from predation control. For example, the Crown-of-Thorns Starfish (Acanthaster planci) increased threefold after a 61% decline in reef fish predators, resulting in a reef dominated by turf algae instead of reef-building corals (Dulvy et al. 2004).
Grouper also display unique collaborative hunting behavior with moray eels. When hungry, the grouper will approach the moral eel with a head-shaking gesture, signaling “Let’s hunt together.” The grouper and moray eel then hunt together to facilitate more frequent prey capture. The large-bodied, slow-moving predators use burst speed and vacuum action of the large buccal cavity to capture fish chased out of crevices of coral reefs (Bshary et al. 2006).
13.5 Fisheries, Management, and Conservation Status of Grouper
Grouper are among the most heavily exploited high-priced reef fish. They have excellent white meat flesh with a light, sweet taste and large chunky flakes that work well with any cooking method. As one of the best ocean fish to eat fresh, grouper are highly sought after by commercial, recreational, and subsistence fishers. They are typically sold fresh in local seafood markets, where they are often the highest-priced fish. They are also part of the live reef fish trade in Southeast Asia, where plate-sized fish may sell for $180 per kilogram.
The annual market value of grouper worldwide has been estimated between U.S. $350 million (Pauly and Zeller 2015) and $1 billion (Sadovy de Mitcheson et al. 2020). However, the economic value of live Nassau Grouper for tourism was 20 times higher than the landed value (Sala et al. 2001). Recreational fishing for grouper is worth hundreds of millions of U.S. dollars in the Gulf of Mexico, where they are often one of the top targets of recreational fishers (Southwick et al. 2016).
Factors such as distance to fish markets and local human population density are often associated with overfishing. Early investigators revealed that many grouper populations displayed signs of both growth overfishing and recruitment overfishing and called for management interventions (Sadovy 1994). Local fishers may assist in instituting restrictions to conserve these most vulnerable populations because they know the time and location of spawning aggregations. Effective management of grouper requires understanding and consideration of their life history and ecological and socioeconomic drivers.
Grouper are caught by gill nets, hook and line, spears, trawls, and traps. There are only a few species that are well studied, and remarkably few official landing records exist for many small-scale grouper fisheries in some tropical and subtropical nations. Lack of detailed catch and effort data makes the assessment of risks of overfishing these valuable fish quite challenging. Larger more economically valuable grouper are often overfished, and fishers switch to harvesting other fish, including smaller grouper species. Partnerships of local fishers and scientists are essential to restore local populations, such as the Nassau Grouper and Atlantic Goliath Grouper. Often the only available information is from recollections of fishers who report that grouper catches were abundant many years ago (Aguilar-Perera et al. 2009; Bender et al. 2014; Amorim et al 2018). Without detailed monitoring, managers must struggle to manage without a fair determination of historical baseline conditions (Bunce et al. 2008; Knowlton and Jackson 2008; Pinnegar and Engelhard 2008).
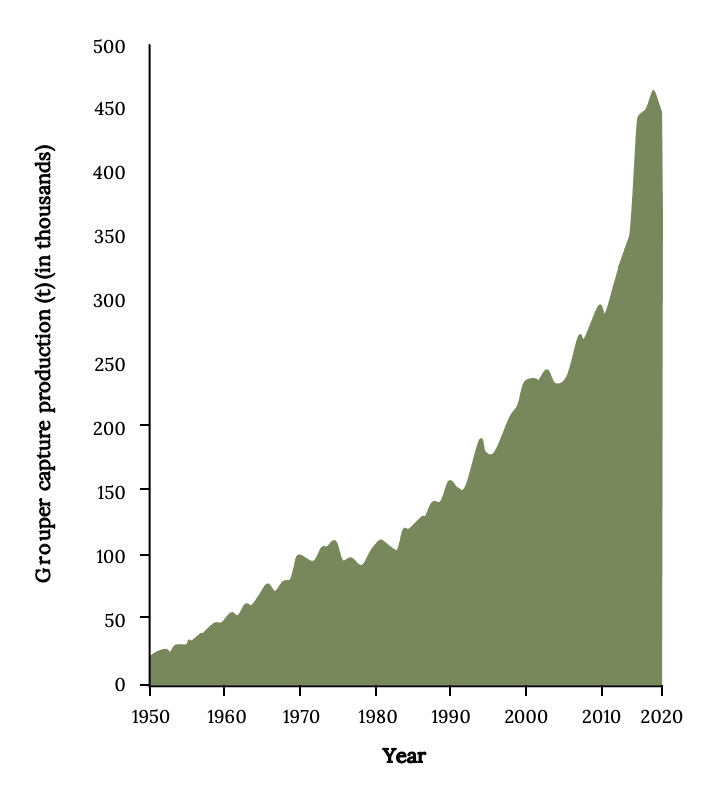
At least 35 different species are harvested to support small-scale, localized commercial and recreational fisheries. Since 1950, the global catches of grouper have increased about 30 times (Figure 13.4). Since the 1980s, most of the catch and the increase was from Asian countries, which accounted for more than 80% of recent landings. Indonesia and China have the largest grouper landings. Many countries that harvest them vastly underreport landings to the FAO. Landings from Cuba, which once had a productive grouper fishery, declined since the 1990s (Claro et al. 2009). Landings in North, Central, and South America are an order of magnitude lower than Asia’s. Therefore, the USA is a net importer of grouper (Sadovy de Mitcheson and Yin 2015). Consequently, as demand increased, many local fishing communities have seen rapid depletion and overfishing (Coleman et al 2000; Sadovy de Mitcheson et al. 2013).
Vulnerability to overfishing is related to ease of capture and a slow life history. For many of the larger grouper species, the combination of slow growth, long life (exceeding four decades), late sexual maturity (up to eight years), and strong site fidelity contribute to this vulnerability. They can easily be approached by divers and captured by spear, hook and line, and even cyanide (Wilcox 2016). Fisheries target adults that are marketed directly for food, as well as juveniles for mariculture grow-out operations (Sadovy and Pet 1998). Catches of many species have declined, and there is “no sign of any slowing down” of declines (Sadovy de Mitcheson et al. 2013). In response to reduced grouper supplies, restaurants often substitute other, less-expensive fish, prompting development of quick assays to identify mislabeled species (Ulrich et al. 2015).
If slow life history and high value create a double jeopardy for grouper, one additional trait creates a triple jeopardy condition. Grouper spawning aggregations, as noted, make them extremely vulnerable at the same time that reproductive values are highest. Furthermore, fishing can cause rapid depletion of sex-changing species due to selection for large adults. Males are usually larger, older, and less numerous than females. Recruitment in grouper is highly variable, and loss of reproductive potential has long-term consequences (Chong-Montenegro and Kindsvater 2022).
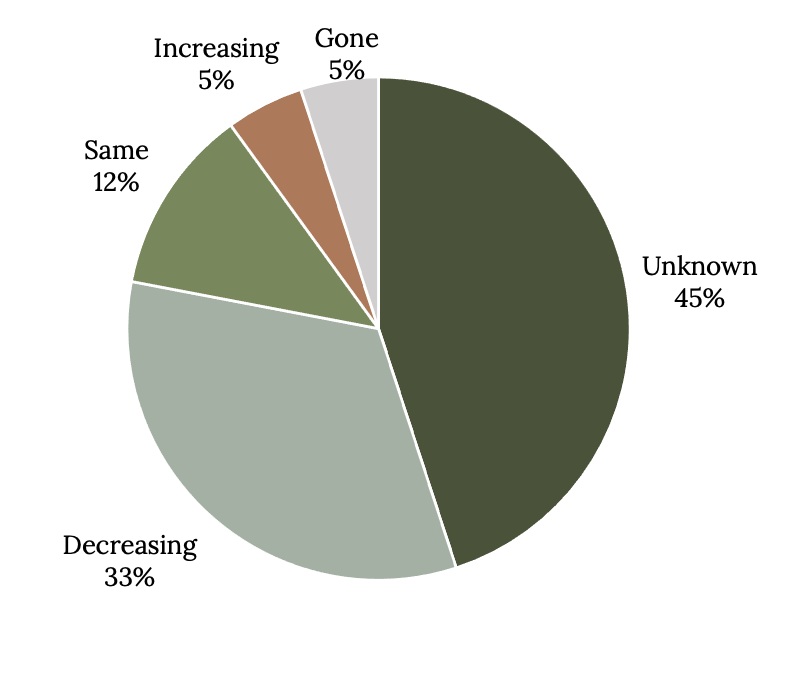
There is no question that fishing is the major factor driving grouper stocks on the downward spiral, but those that have large spawning aggregations are most vulnerable to declines (Coleman et al. 1996; Asch and Erisman 2018; Sadovy de Mitcheson et al. 2020). Because it takes a long time for scientists to obtain needed life history information, fisheries-independent survey data, and catch history, grouper populations may be overfished long before data are even available for a stock assessment. Without formal stock assessments, general indicators of population status are based on catch trends. Very few grouper stocks that have spawning aggregations are managed sustainably. In a recent global analysis of the status of populations that form spawning aggregations, 45% were unknown, 33% were decreasing, and 5% were already gone (Figure 13.5). Only 12% had stable populations, and 5% were increasing.
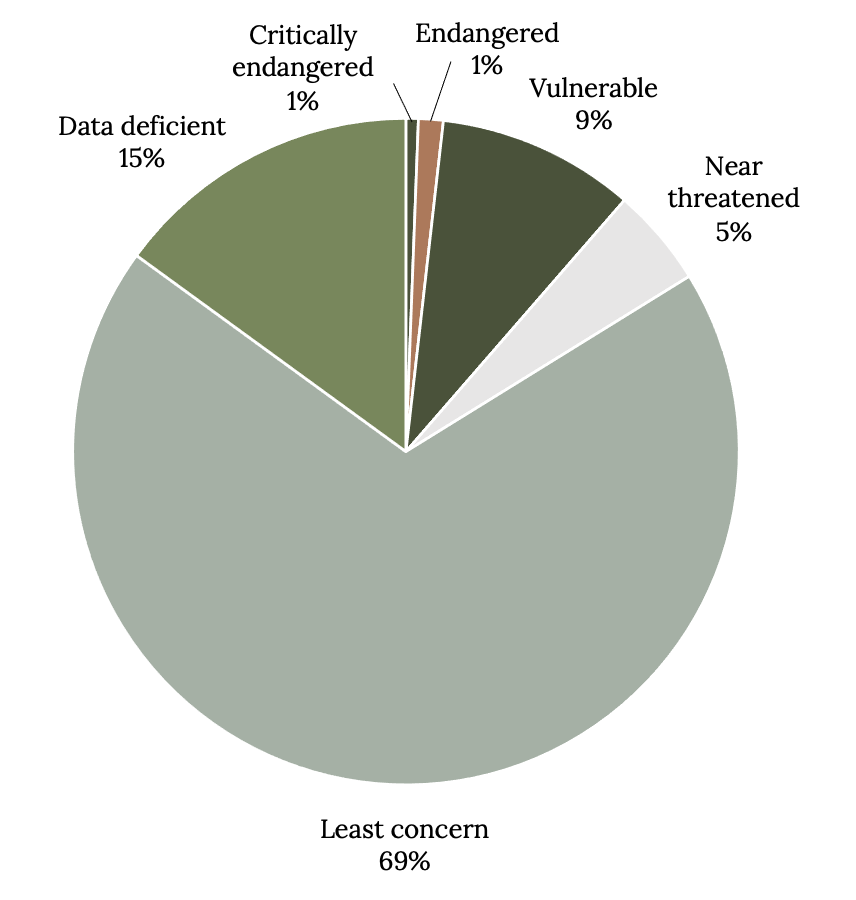
Of the 167 species of grouper, 9.6% are vulnerable, 4.8% are near threatened, 1.2% are endangered, and 0.6% are critically endangered (Figure 13.6). The majority of species (68.9%) are classified as least concern and 15% are data deficient, with insufficient data for classification. The larger (>50 cm total length) and long-lived (>20 years) species of grouper that also had smaller geographic ranges were most likely to be endangered or critically endangered (Luiz et al. 2016). Market prices for grouper are escalating, and other lower-valued fish are often mislabeled or substituted.
To protect grouper from overfishing, many measures are being implemented, such as minimum and slot-size limits, recreational bag limits, commercial fishing quotas, gear and seasonal controls, marine protected areas, and limited entry (Rocklin et al. 2022). The effectiveness will depend on traits of the species and the local context. Regulations to prevent marketing of undersize fish will mitigate growth overfishing. Allowing smaller fish to reach maturity at least once before harvest will mitigate recruitment overfishing. Size-limit regulations focused on protecting spawning-size fish may be ineffective for deepwater recreational fishing. Grouper have a physoclistous (i.e., closed) swim bladder, making them particularly susceptible to ruptured swim bladders, bloating, stomach distention, and protruding eyes caused by rapid decompression when hauled to the surface (Brulé et al. 2015). The proportion of grouper with distended stomachs was 70% in one study of commercial hook-and-line fishing and as high as 95% for Red Grouper in water deeper than 41 m (Bacheler and Buckel 2004). Consequently, minimum size limits may be ineffective regulations (Rudershausen et al. 2007).
Lack of data collection for many species of grouper leaves important knowledge gaps that prevent effective management. Identifying and protecting sites of known spawning aggregations with closed seasons are recommended to prevent the rapid declines or allow for population recovery (Coleman et al. 2000). Since experienced local fisheries can detect the declines in grouper abundance, the locations of aggregations are often known. No-take marine fishery reserves represent a viable means to protect resources while simplifying enforcement. Grouper show significant increases in size and biomass within no-take marine protected areas (MPAs), especially for smaller and medium-sized species and those that do not migrate (Chiappone et al. 2000; Nemeth et al. 2005; Howlett et al. 2016; Erisman et al. 2017; Belharet et al. 2020; Chollette et al. 2020; Rojo et al. 2021). It takes a long time for them to recover to preharvested levels after full protection, often 20 or more years (Russell et al. 2012).
Because some grouper populations have been exploited for millennia, it is a challenge to establish realistic conservation targets (Guidetti and Micheli 2011). Large individuals are often rapidly extirpated from shallow reefs and restricted to deep waters. Coastal fishers usually have detailed knowledge on diet and trophic relationships of exploited fish (Ribeiro et al. 2021). Often the local ecological knowledge of coastal fishers is the only source of information on sites of historical spawning aggregations.
Question to ponder:
Compare and contrast the life history traits of Pacific Salmon with those of grouper. Which traits make each group particularly vulnerable to overfishing?
13.6 Live Reef Fish Trade
A specialty at many top restaurants in some Asian countries is live fish for the consumer to select for their menu item. The live reef food fish trade has a long history, but it has grown substantially since the 1990s as the number of superaffluent people in Asia grows. Improved airline connections also spurred the expansion, allowing for the more rapid transport necessary for live animals. The destination for the live reef fish trade is centered in Hong Kong, which has more billionaires than any other city (Philips et al. 2008). In 2017, the financial center of Hong Kong posted rapid growth in its ultrawealthy population to overtake the New York metropolitan area as the world’s largest ultrawealthy city (Wealth-X 2018). The Asian affluent are outgrowing the conventional definitions of luxury. It’s not just about owning luxury materials but often more importantly experiencing it—often before others do. Plate-sized live grouper held for sale at restaurants are examples of what economists call Veblen goods (Veblen 1912). Unlike normal supply-demand relationships, even as the price of Veblen goods increases, the demand increases. High prices associated with certain size classes and species may make it worthwhile for fishermen to focus their fishing effort on that size class.
Harvest of live grouper to meet the demands of the live reef fish trade is primarily in the Coral Triangle region (Sadovy de Mitcheson 2019). This is one of the most important reef systems in the world, encompassing Indonesia, Malaysia, the Philippines, Papua New Guinea, the Solomon Islands, and Timor-Leste. Indonesia and the Philippines are the largest exporters of grouper (Khasanah et al. 2020). The coral reef fishers learn to “free the size that does not pay” and are able to be very selective by choice of hook, location, and depth. The supply chain for the live reef fish trade is not well monitored from the fisher to first buyers, exporters, importers, wholesalers, retailers, and consumers. Up to 80% of the live fish on sale may be juveniles, and many larger species are rare (To and Sadovy de Mitcheson 2009).
The growing demand for live grouper has increased the interest in capture-based culture of the fish. Here, large numbers of juveniles are harvested and raised in cages to the most profitable size (Pierre et al. 2008). This type of fish culture depends on unchecked harvest of juvenile grouper, and the resulting fisheries are likely to be unsustainable (Sadovy de Mitcheson and Liu 2008; To and Sadovy de Mitcheson 2009). The unfortunate reality is that the demand for live grouper for international trade far outstrips the sustainable supply (Sadovy et al. 2003). Strong enforcement of fishing regulations is lacking, and underreporting of harvest is common.
Whether this unique market demand affects profits or fish populations depends on biology, particularly the sex and maturity of the target size. Many grouper populations near Hong Kong were virtually extirpated, forcing suppliers to seek fish from distant locations. Market-driven, size-selective fishing can result in decreases in the catch of large, disproportionately fecund fish—the big old fat fertile female fish (BOFFFFs)(Reddy et al. 2013). A fishery that targets large grouper would influence male abundance, leading to concerns of sperm limitation on productivity (Koenig et al. 1996; Heppell et al. 2006). However, a fishery that targets the plate-size grouper (20–40 cm) is taking subadult fish, which can quickly extirpate local populations (Reddy et al. 2013; Kindsvater et al. 2017). Therefore, these fisheries need strong enforcement of regulations limiting catch of juveniles and adults. While lucrative fisheries target live reef fish markets (Sadovy de Mitcheson et al. 2017), overfishing by harvesting juveniles threatens the livelihoods of many who rely on fish as their primary protein source.
Questions to ponder:
In what ways are the marine tropical fish trade similar to the live reef fish trade? Are there similarities in approaches to regulate these two industries?
13.7 Culture of Grouper
High prices paid for plate-sized grouper and a short culture time have driven many Asian countries to invest in culture facilities (Pierre et al. 2008; Tupper and Sheriff 2008). At least 47 species of grouper are raised in culture grow-out pens and fed until they reach a marketable size (Rimmer and Glamuzina 2019). Full life-cycle aquaculture is not yet possible for most species. Rather, juveniles are harvested from the wild at sizes ranging from 2 to 112 cm (Sadovy and Pet 1998).
Mass production of fry from Giant Grouper was first achieved in Taiwan in 1996 and was soon followed by other Asia-Pacific countries. In Taiwan, grouper production depends on hatcheries for approximately two-thirds of its output. Milt from Giant Grouper has been used to fertilize eggs of Tiger Grouper to produce a hybrid (Tiger Grouper ♀ × Giant Grouper ♂). The hybrid has improved growth rate. In Vietnam, hybrid grouper is the second-most-important crop for nursery farms due to strong market demand and sales prices, fast growth rate, and higher survival compared to other grouper crops (Dennis et al. 2020).
Grouper farms employ numerous workers for spawning, larval rearing, and grow-out phases of their operations. The largest production comes from China, Taiwan, and Indonesia (Rimmer and Glamuzina 2019). Although some farms use formulated feed, many still rely on harvesting other marine fish to feed grouper. Disease outbreaks are common and result in reduced survival to market size. Culture of grouper does not reduce fishing pressure on them, and millions of fishers globally will continue to depend on wild capture. The process is a relatively new venture, and prospects are still uncertain (Sadovy and Lau 2002). Yet, recent data shows that about 50% of live grouper imported to Hong Kong are from fish farms (Rimmer and Glamuzina 2019). Future advances in selection of improved strains, first foods, feed formulation, full-life-cycle hatcheries, and water quality enhancements are expected.
13.8 Case Study: Nassau Grouper
The Nassau Grouper (Epinephelus striatus) is the most important finfish in The Bahamas and valued culturally, economically, and ecologically. It occurs in rocky bottoms and coral reefs in over 30 countries and territories from the Gulf of Mexico and along the tropical western Atlantic and Caribbean south to Brazil. The name striatus refers to the pattern of light background and irregular dark brown bars, which helps it blend into its habitat (Figure 13.7). People in The Bahamas rely on Nassau Grouper as an important food as well as a target for a thriving dive and tourism industry. Grouper supported many Bahamians for centuries, providing over $1 million in landings per year. Nassau Grouper is the essential ingredient in the local comfort food, Bahamian boiled fish, or simply “boil,” which is eaten for breakfast, lunch, or dinner.

Nassau Grouper were once plentiful across shallow coastal zones of Bermuda, Florida, The Bahamas, the Yucatán Peninsula, and throughout the Caribbean. The first-ever eye-witness account described a spectacular gathering of 30,000 to 100,000 large adult Nassau Grouper (Lavett-Smith 1972). Despite an increase in observers, this observation remains the largest grouper aggregation ever recorded. In a six-day survey of this same site 40 years later, only five Nassau Grouper were observed (Erisman et al. 2013). As early as the 1990s, available evidence showed that Nassau Grouper were overfished and many spawning aggregations had disappeared (Sadovy 1994; Aguilar-Perera and Aguilar-Dávila 1996; Chiappone et al. 2000; Claro and Lindeman 2003; Claro et al. 2009; Aguilar-Perera 2014). The collapse of the Nassau Grouper throughout its range was due to overfishing on spawning aggregations (Sadovy and Eklund 1999; Sala et al. 2001; Aguilar-Perera 2006). Historical landing records in The Bahamas and elsewhere show that much of the annual harvests of Nassau Grouper was taken from spawning aggregations during the winter months. The population decline resulted in a drop in commercial landings of 86% over the past 20 years (Sherman et al. 2016). Over 60 Nassau Grouper spawning aggregation sites were identified globally, but many of these have been lost due to overfishing (Sadovy and Eklund 1999; Sadovy de Mitcheson et al. 2008). Nassau Grouper is classified as endangered by the International Union for the Conservation of Nature (Bertoncini et al. 2018) and is listed as threatened under the U.S. Endangered Species Act (81 FR 42268, June 29, 2016).
Nassau Grouper are solitary reef dwellers. However, mature individuals migrate during the full moon to spawning aggregation sites. Movements of Nassau Grouper are highly synchronized to specific spawning sites at predictable times (Bolden 2000; Starr et al. 2007; Stump et al. 2017). First, they leave territories in shallow water near winter full moon, then migrate to their spawning site in water ~100 m deep (Washckewithz and Wirtz 1990). Synchronization is helped by sounds produced by migrating Nassau Grouper (Hazlett and Winn 1962; Rowell et al. 2015). One explanation for the consistency of migration routes and spawning locations is that younger fish learn migration routes from more experienced migrators and their unique sounds. Different color patterns develop when Nassau Grouper are ready to spawn. A bicolor pattern indicates a nonaggressive submissive state acquired by both males and females near the time of spawning. The dark phase is acquired by females who are followed by numerous bicolor fish during courtship (Colin 1992). The courtship occurs in late afternoon, followed by a spawning rush near sunset, where the bicolor female swims upward and releases eggs while the males follow behind releasing sperm (Sadovy and Eklund 1999).
A mix of habitats is important for the life cycle of the Nassau Grouper. After spawning in deep water, their fertilized eggs float and reach the surface within three to five hours of spawning, and newly hatched embryos are also positively buoyant within two to three days after hatching (Colin 1992). Wind-driven currents likely influence the transport of small larvae during the first days after spawning. Larval Nassau Grouper are adapted for life in near-surface waters and have elongated dorsal spines that resemble small underwater kites. Larvae feed on plankton for 35 to 40 days before settling in seagrass meadows, macroalgal beds, or mangrove nursery habitats. Juvenile Nassau Grouper may be supported by feeding on crabs from adjacent seagrass beds (Eggleson et al. 1998). As the young grow, they move to offshore reefs.
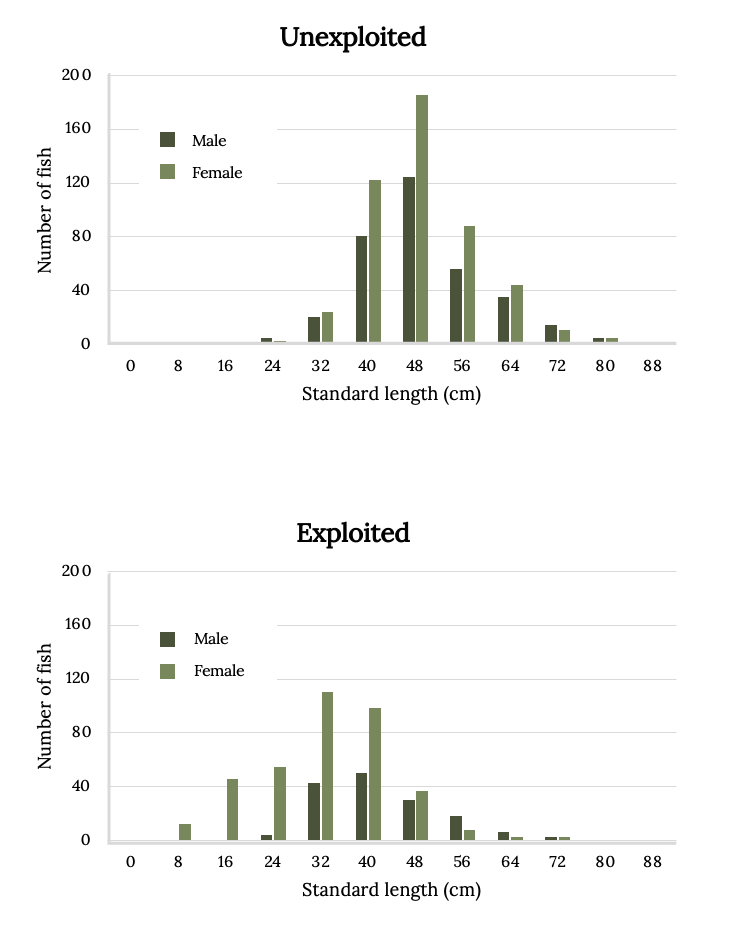
Belize was one of the first countries to protect the Nassau Grouper via closed fishing seasons at sites of spawning aggregations. The effect of seasonal closures is evident in comparison of size distributions of exploited sites with unexploited sites (Figure 13.8). Nassau Grouper begin to mature at approximately 48 cm in length, and by the time they reach 56 cm, 75% are mature (Carter et al. 1994; Sadovy and Colin 1995, Sadovy and Eklund 1999). Fishing has eliminated many of the largest and most fertile individuals (Figure 13.8).
In The Bahamas, the fishing industry contributes approximately $85 to 90 million annually, with Nassau Grouper sales of approximately $1.5 million. Nassau Grouper populations are much more abundant in the Exuma Cays Land and Sea Park, where all fishing has been prohibited
since 1986. Protection of their spawning aggregations began in 1998 with seasonal closures of two sites during the winter months. During the closed season, the capture or sale of Nassau Grouper is prohibited. Beginning in 2004, the closed season was extended countrywide. By 2010, a majority of the fishers (82%) still had concerns about the future of The Bahamas’ Nassau Grouper fishery, as the catch per day remained low (Cheung et al. 2013). Problems with enforcing the seasonal closure and poaching, as well as the introduction of air compressors by spear fishers, meant that they remained overfished in The Bahamas. Existing management measures, such as the small 3-pound (1.4-kg) size limit and noncompliance with fishing regulations in The Bahamas, likely prevent recovery of these fish (Sherman et al. 2016). Tourist visitation effectively stopped during the COVID-19 pandemic from spring through the autumn of 2020, resulting in an increase in large Nassau Grouper in one marine protected area (Kough et al. 2022).
In 1985, the Cayman Island government, responding to fishermen’s concerns over declining numbers and size of Nassau Grouper, restricted fishing on five known spawning aggregations to only residents using hook-and-line gear. In 2003, the government passed legislation to establish no-take during spawning months and bag and slot limits away from aggregation sites in the rest of the year to allow recreational and artisanal catch outside the spawning season.
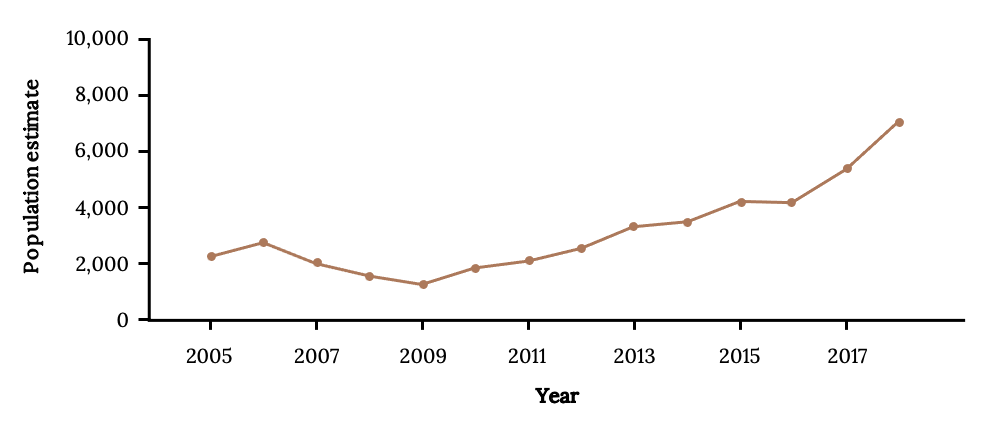
The protections initiated by the Cayman Islands government resulted in sustained recovery of a population of Nassau Grouper previously on the brink of extirpation (Figure 13.9). More individuals are larger than 65 cm, and spawning biomass and recruitment have increased (Stock et al. 2021). Little Cayman now has the largest-known spawning aggregation for Nassau Grouper, and Cayman Brac is markedly improved (Sadovy de Mitcheson 2020; Waterhouse et al. 2020). Management interventions to safeguard the Little Cayman spawning aggregation provide other countries a ray of hope for grouper recovery.
Strict regulations on fishing can diminish livelihoods of subsistence fishers. Dive tourism may provide alternative livelihoods and mitigate the negative effects of closures for displaced fishers (Sala et al. 2001; Heyman et al. 2010; Usseglio et al. 2016). To learn more about the incredible long-term work underway in the Cayman Islands to protect the Nassau Grouper as part of the Grouper Moon Project, watch the video https://youtu.be/TfsUsCgCH0A.
13.9 Case Study: Goliath Grouper
The Goliath Grouper is the largest grouper in the Atlantic Ocean and one of the two largest species of grouper in the world, reaching ~2.5 m (7–8 ft) in total length. In the western Atlantic Ocean, it ranges from North Carolina to southern Brazil, including the Gulf of Mexico and the Caribbean Sea. Advertisers tout Florida as the only place in the world where Goliath Grouper can be found on a regular basis throughout the year and in their spawning aggregation sites in late summer. Goliath Grouper were intensively overfished long before landing records were kept so that old photographs from fishing marinas provide hints to the past (Figure 13.10). They have several traits that make them vulnerable to overfishing, including high longevity, late maturation, site fidelity, aggregative spawning, and a lack of fear of humans (Sadovy and Eklund 1999).
The largest grouper ever caught and certified by the International Game Fishing Association was a 680-pound (309-kg) Atlantic Goliath Grouper. This record fish was taken off the coast of southern Florida in 1961 after decades of overfishing (McClenachan 2009). One analysis revealed that increasing fishing effort and widespread use of fish finders reduced the abundance of adults to only 5 to 10% of virgin levels (Porch et al. 2006). The Goliath Grouper has been severely overfished throughout its range, and a fishing moratorium was initiated in U.S. and state waters in 1990 and throughout the Caribbean in 1993 (Aguilar-Perera et al. 2009). In the Caribbean Sea in Mexico and Belize, few people even remember the presence of this giant fish (Graham et al. 2009; Bravo-Caldero et al. 2021). Similarly, in Brazil even low levels of spearfishing led to depletion of Goliath Grouper, which are considered functionally extinct despite a ban imposed in 2002 (Giglio et al. 2017).

Goliath Grouper live at least 37 years or more and reach sexual maturity after four years (males) and six years (females) (Bullock et al. 1992). Each year, they migrate to gather in reproductive aggregations of up to 100 individuals. They spawn during the summer (January to March) in the Southern Hemisphere, similar to summer spawning (July to September) in the Northern Hemisphere. Sounds produced serve to synchronize timing of migration (Mann et al. 2009). Juveniles and adults often return to the same site to spawn year after year, making them particularly susceptible to overfishing (Colin 1994). Spawning occurs at night, presumably to avoid egg predation by opportunistic egg predators, such as scad and herring. In many regions, the spawning aggregations are known only from anecdotal recollections by veteran fishers, and others have disappeared without having been documented (Aguilar-Perera et al. 2009; Bueno et al. 2016).
The Origins of the Atlantic Goliath Grouper Common Name
Scientists who describe new species are responsible for giving it a valid Latinized binomial name. According to the International Code of Zoological Nomenclature (ICZN), the first part identifies the genus to which the species belongs, and the second part identifies the species within the genus. Only scientific names are covered by the ICZN. Common, or vernacular, names often vary among regions. In North America, common names are standardized by a committee of the American Society of Ichthyologists and Herpetologists (ASIH) and the American Fisheries Society (AFS). The common name for Epinephelus itajara was formerly the jewfish. I observed my first jewfish in the John G. Shedd Aquarium when I was a young boy. I thought it was a strange and nondescriptive name for such a ginormous fish. The historical origins and meaning of “jewfish” are unclear because scientists did not have to explain common names when describing fish species. A story that jewfish were so named because they were an inferior fish, fit only for Jews, persisted since the 1800s (Grossman 2015). The Common and Scientific Names Committee of AFS received complaints about the offensive jewfish name, as well as the squawfish name, derogatory toward women. The Names Committee changed the accepted common name of the squawfish to the pikeminnow in 1998. Soon complaints about the jewfish name led to a formal petition signed by senior fisheries scientists sent to the committee. Clearly names and their meaning have tremendous power, and associating Jews with a large-jawed grouper extended to members of the Jewish faith. After committee deliberations in 2001, they declared that the new accepted common name would be Atlantic Goliath Grouper.
Although most fishing for Goliath Grouper is offshore near reefs and structures, the species is mangrove dependent and shows a distinct size-related habitat shift. Juveniles are found exclusively in spatially complex, fringing Red Mangrove (Rhizophora mangle) shorelines (Frías-Torres 2006; Koenig et al. 2007). The mangrove forests support a high diversity of fish and invertebrates and are threatened worldwide. Mangroves create a narrow fringe habitat between land and sea at tropical latitudes (25ºN to 30ºS). Since 1980, at least 35% of mangrove forests were lost to coastal development (Valiela et al. 2001). High-quality mangrove habitat in southwest Florida is the key to recovery (Frías-Torres 2006; Koenig et al. 2007; Koenig and Coleman 2009). Juveniles spend their first five to six years of life in mangroves, and it was here in the juvenile population that the first signs of recovery appeared (Cass-Calay and Schmidt 2009).
The functional extinction of the critically endangered Atlantic Goliath Grouper in many parts of the range has attracted much attention, and fishing moratoria are common. Recovery of populations depends on conditions in nursery areas (Koenig et al. 2007; Shideler et al. 2015b; Lobato et al. 2016) and far-distant spawning aggregations. Research that combines local ecological knowledge and takes advantage of technologies, such as bioacoustics, biotelemetry, sonar, and remote and autonomous underwater vehicles, may lead to more accurate information on grouper spawning aggregations (Erisman et al. 2017). Photo-identification is widely used for noninvasive mark-recapture analysis and appears to be well suited for the sedentary, large Goliath Grouper in marine parks frequented by divers (Hostim-Silva et al. 2017).
Recovery of the critically endangered Atlantic Goliath Grouper will require actions to (1) protect coastal lagoons with fringing mangrove nursery areas; (2) locate spawning aggregations and learn from traditional ecological knowledge; (3) adopt large no-take protected areas and evaluate diving tourism alternatives (Heyman et al. 2010; Shideler and Pierce 2016); and (4) halt poaching (Giglio et al. 2014). However, given the strict nature of regulations needed, leadership, social networks, and comanagement at the local level are often the glue that will make these conservation plans successful (Gutiérrez et al. 2011).
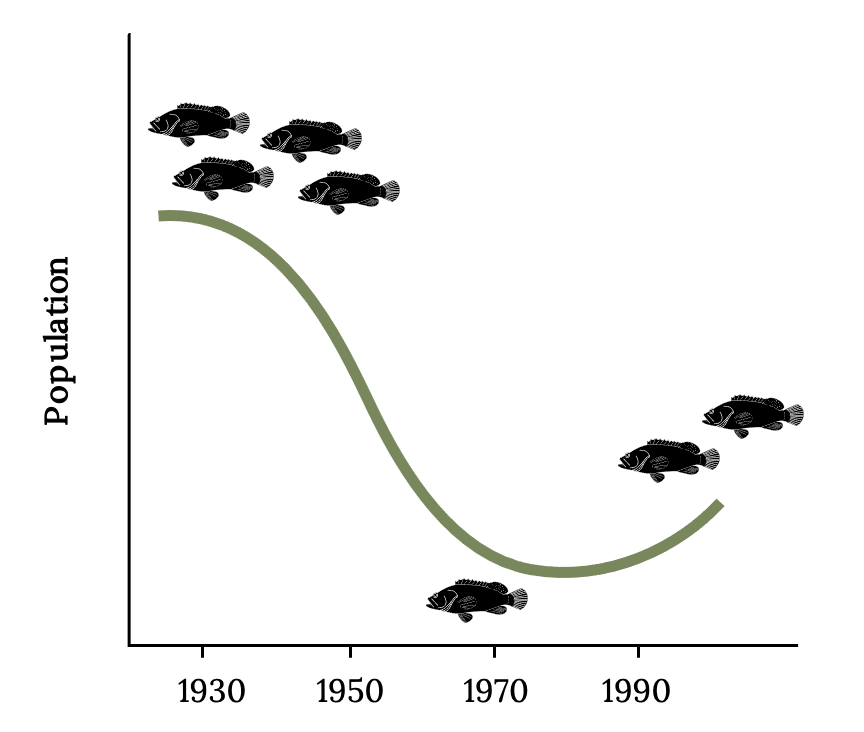
There are signs of recovery in Florida waters after thirty years of a fishing moratorium on Atlantic Goliath Grouper (Figure 13.11; Koenig and Coleman 2011). Grouper represent only one of many valuable residents of threatened coral reef ecosystems. Restoring coral reef ecosystems will require reducing and reversing carbon emissions that are driving global climate change (Knowlton and Jackson 2008).
While full recovery is still uncertain, sport fishers are aware of the increase in large Goliath Grouper. Return of a spawning aggregation near Jupiter, Florida, is one encouraging sign of recovery (Frías-Torres 2013). Many people unfamiliar with the history of changes in Florida reefs now consider Goliath Grouper to be novel and intolerable because of the moratorium on fishing for them. Some recreational anglers called for the lifting of the fishing moratorium. However, the reasons given to support this petition (below) are not supported by scientific evidence (Koenig et al. 2020).
False claims in support of lifting the moratorium:
- Goliath Grouper compete directly with recreational reef fish fishermen for and substantially reduce the populations of grouper and snappers on reefs in south Florida.
- Goliath Grouper are dangerous to divers.
- Goliath Grouper interfere with fishing by taking baited hooks, or hooked or speared fish.
- Goliath Grouper compete directly with lobster fishermen by eating many lobsters in south Florida.
- Goliath Grouper, because of their large size, require huge amounts of food to survive and eat indiscriminately, reducing biodiversity on reefs.
- Our reefs are “out of balance”; Goliath Grouper have to be “thinned out” to regain that balance.
- There must be a periodic kill of hundreds of adult Goliath Grouper to obtain data on size, age, and reproductive condition necessary for stock assessment.
Furthermore, Goliath Grouper hold the unfortunate distinction of having the highest levels of liver and muscle mercury of any commercially important shallow-water grouper species. Mercury levels in the muscle tissues of most adults and many juveniles from Florida samples exceeded safe levels for human consumption (Malinowski 2019). The large size of Goliath Grouper adds to the interest and pressure by sport anglers to lift the current harvest moratorium on them. Divers and scientists, however, oppose lifting the moratorium. Despite opposition by scientists and divers, Florida officials lifted the Goliath Grouper ban in 2022 (Collins 2022). The new rules prohibit spear fishing and limit annual harvest to 300 fish between 24 and 36 inches. Time will tell if the fishery is sustainable.
Grouper spawning aggregations are also a strong draw for SCUBA divers in many popular tourist destinations, including Palau, Belize, and French Polynesia. Consequently, future developments may focus on creating tourist adventures based on diving with grouper. Divers are willing to pay more for Goliath Grouper encounters (Shideler and Pierce, 2016), making them more valuable as diving attractions than for harvest (Shideler et al. 2015a).
Question to ponder:
What do you suspect are the principal reasons for opposing the creation of no-take marine reserves to protect grouper populations?
Profile in Fish Conservation: Yvonne Sadovy de Mitcheson, PhD

Yvonne Sadovy de Mitcheson has been Professor at the University of Hong Kong for 30 years and is well known as the foremost expert on grouper conservation and ecology. She teaches many courses that deal with the biology, fisheries management, and conservation of fish. Her research and scholarly writings leave an important global legacy, providing a roadmap for conservation and fisheries management of grouper and other marine fish.
Dr. Sadovy’s early studies were based in the Caribbean and represent many of the first investigations into the exploitation of sex-changing coral reef fish, especially grouper that form spawning aggregations. Her first investigations in this region revealed that many grouper populations were overfished and that the monitoring and assessment activities were inadequate. For five years, she served as the Director of the Fisheries Research Laboratory of the government of Puerto Rico and then as biologist with the Caribbean Fishery Management Council of the National Marine Fisheries Service (NOAA, USA).
She is the author or coauthor of more than 160 publications that investigate the biology and conservation of marine fish, with particular emphasis on the grouper and other reef fish vulnerable to fishery exploitation. Studies that focus on the trade in live tropical food and ornamental fish, locally, regionally, and globally, revealed several global threats from fishing. Additionally, she and her collaborators added significantly to our knowledge of reproduction, including sex differentiation, maturation and gonadal development, and age and growth of many reef fish. She spearheaded investigations of the live reef fish markets and trade in Hong Kong and their role in supporting imports of many highly valued species, several of which are threatened. Her efforts led to adoption of scientific protocols for documenting and monitoring fished and unfished grouper spawning aggregations throughout the world.
Yvonne’s keen interest in public education on marine conservation issues has expanded the impact of her studies to effect awareness and facilitate policy changes. Professor Sadovy de Mitcheson founded and is currently co-Chair of the IUCN World Conservation Union Specialist Group on Grouper and Wrasses. She shares her expertise with conservation groups such as the World Wide Fund for Nature Hong Kong, Wildlife Conservation Society, TRAFFIC–East Asia, and the Food and Agriculture Organization of the United Nations. She is Director of the Science and Conservation of Reef Fish Aggregations, a nonprofit organization that seeks to raise awareness about the vulnerabilities of fish spawning aggregations and improve their protection and management. This collaborative effort resulted in books including Reef Fish Spawning Aggregations: Biology, Research and Management, Manual for the Study and Conservation of Reef Fish Spawning Aggregations, and training modules. She coauthored Groupers of the World: A Field and Market Guide, which is a comprehensive and colorful description of over 150 species of grouper.
Recent research efforts focused on the development of a scientific model for sustainable exports of the endangered Napoleon Wrasse (Cheilinus undulatus), the largest coral reef species and a part of the live reef food fish trade. Her early work on the Nassau Grouper in the tropical western Atlantic was a major impetus for sustainable management planning, and she recently completed a major management plan on this species for FAO. Recently, she has investigated the threats and opportunities for the growing international demand for dried swim bladders and is leading a team to develop a facial recognition app to aid enforcement in the trade for the Napoleon Wrasse.
Key Takeaways
- Large body size, slow growth, high longevity, late reproductive maturity, and the reproductive behavior of forming spawning aggregations all contribute to the vulnerability of grouper stocks.
- Length or creel limits are often ineffective for grouper in deep waters, where they develop barotrauma after deepwater capture.
- Long time periods are required to recover larger grouper species, such as the Goliath Grouper and Nassau Grouper.
- Local management interventions may include bans on fishing during reproductive seasons, marine protected areas, shift to grouper tourism via SCUBA diving, and adopting international standards for the trade in international live reef food fish.
- Poor fisheries governance structures are in place in less-developed countries, and many grouper stocks are data deficient.
- Protective management actions will take decades to evaluate because of the long time to maturity and long recovery times for grouper.
- Goliath Grouper were protected in Florida waters by a fishing moratorium since 1990, and signs of recovery are emerging.
This chapter was reviewed by Felicia Coleman.
Long Descriptions
Figure 13.1: Red Grouper with robust body and small scales. Their head and body are dark reddish brown, shading pink or reddish below with occasional white spots on the sides and black spots on the cheeks. Jump back to Figure 13.1.
Figure 13.2: Diagram of habitats used at different life stages for Goliath Grouper; 1) post larvae settle in mangrove litter and roots; 2) juveniles hide in mangrove microhabitats; 3) older juveniles migrate to coral reefs; 4) adults live on reefs for 40+ years; 5) adults migrate and spawn into water column; 6) fertilized eggs drift in currents; 7) larvae hatch from eggs and drift in currents for 30-80 days. Jump back to Figure 13.2.
Figure 13.3: Length (cm) on x-axis and trophic level on y-axis. From smallest length (0.01 cm) and lowest trophic level to longest length (1000 cm) and highest trophic level: Decomposers, Microplankton, crustacean zooplankton, gelatinous zooplankton, planktivores, intermediate predators, top predators. Eggboons point to all groups except for predators. Jump back to Figure 13.3.
Figure 13.4: X-axis shows years from 1950-2020. Y-axis shows grouper capture production (t) in thousands from 0 to 500. Increases consistently with a higher rate of increase from 2010-2020. Jump back to Figure 13.4.
Figure 13.5: Pie chart shows that status of exploited grouper aggregations is often unknown or declining; gone (5%), unknown (45%), decreasing (33%), same (12%), increasing 5%. Jump back to Figure 13.5.
Figure 13.6: Pie chart shows conservation status of groupers; data deficient (15%), critically endangered (1%), endangered (1%), vulnerable (9%), near threatened (5%), least concern (69%). Jump back to Figure 13.6.
Figure 13.7: Nassau grouper with large eyes and a robust body. Light beige with five dark brown vertical bars, a large black saddle blotch on top of the base of the tail, and a row of black spots below and behind each eye. Jump back to Figure 13.7.
Figure 13.8: Two bar graphs. Standard length (cm) on x-axis from 0-85. Number of fish on y-axis from 0-200. Top graph: Unexploited. There are more females than males for almost every length. 48cm have the most fish (170). Bottom graph: Exploited. There are more females than males for almost every length. 32cm have the most fish (120). Jump back to Figure 13.8.
Figure 13.9: Line graph shows population estimate for Nassau Grouper at Little Cayman from 2005 to 2018. Population grows from 2,000 in 2005 to 7,000 in 2018. Population decreased from 2006-2009. Jump back to Figure 13.9.
Figure 13.11: Diagram of goliath grouper population from 1930-2000. Population is highest in 1930, declines until 1970, then starts to increase again until 2000. Jump back to Figure 13.11.
Figure References
Figure 13.1: Red Grouper (Epinephelus morio) is commonly caught by recreational and commercial fishers from southern Brazil to North Carolina, including the Gulf of Mexico and Bermuda. Simões et. al., 2014. CC BY 4.0. https://commons.wikimedia.org/wiki/File:Epinephelus_morio_in_Madagascar_Reef.jpg;
Figure 13.2: Conceptual diagram illustrating the Goliath Grouper life cycle and movement of various life stages throughout the nearshore and reef environments. Kindred Grey. 2022. CC BY-SA 4.0. Adapted from Goliath Grouper Life Cycle, by Jane Hawkey, Integration and Application Network, https://ian.umces.edu/media-library/goliath-grouper-life-cycle/. CC BY-SA 4.0.
Figure 13.3: Flow energy from grouper eggs to components of the coral reef ecosystem (solid arrows) and trophic transfers through the food web (dashed arrows). Kindred Grey. 2022. CC BY-SA 4.0. Adapted from Egg Boons: Central Components of Marine Fatty Acid Food Webs, by Fuiman et. al., 2015. https://doi.org/10.1890/14-0571.1. Includes Mixed Phytoplankton Community Coloured by Tracey Saxby, Integration and Application Network from https://ian.umces.edu/media-library/mixed-phytoplankton-community-coloured/ (CC BY-SA 4.0), Feather duster worm by Diana Kleine, Marine Botany UQ from https://ian.umces.edu/media-library/feather-duster-worm/ (CC BY-SA 4.0), Paraclanus spp by Kim Kraeer, Lucy Van Essen-Fishman, Integration and Application Network from https://ian.umces.edu/media-library/paraclanus-spp/ (CC BY-SA 4.0), Panulirus argus (spiny lobster): side view by Caroline Donovan, Integration and Application Network from https://ian.umces.edu/media-library/panulirus-argus-spiny-lobster-side-view/ (CC BY-SA 4.0), Moerisia spp. (Jellyfish) by Tracey Saxby, Integration and Application Network from https://ian.umces.edu/media-library/moerisia-spp-jellyfish/ (CC BY-SA 4.0), Chromis chromis (Mediterranean Chromis) by Tracey Saxby, Integration and Application Network from https://ian.umces.edu/media-library/chromis-chromis-mediterranean-chromis/ (CC BY-SA 4.0), Carcharhinus plumbeus (Sandbar Shark) by Tracey Saxby, Integration and Application Network from https://ian.umces.edu/media-library/carcharhinus-plumbeus-sandbar-shark/ (CC BY-SA 4.0), Oncorhynchus tshawytscha (Chinook Salmon): adult by Emily Nastase, Integration and Application Network from https://ian.umces.edu/media-library/oncorhynchus-tshawytscha-chinook-salmon-adult/ (CC BY-SA 4.0), Eubalaena glacialis (Right Whale) by Jamie Testa, Integration and Application Network from https://ian.umces.edu/media-library/eubalaena-glacialis-right-whale/ (CC BY-SA 4.0), and Turtle eggs by Kim Kraeer, Lucy Van Essen-Fishman, Integration and Application Network from https://ian.umces.edu/media-library/turtle-eggs/ (CC BY-SA 4.0).
Figure 13.4: Grouper capture fisheries catches reported to FAO from 1950 to 2018. Kindred Grey. 2022. CC BY 4.0. Data from The Importance of Grouper and Threats to Their Future, by Yvonne Sadovy de Mitcheson and Min Liu Biology, in Ecology of Grouper, 2022. https://doi.org/10.1201/b20814.
Figure 13.5: Current known status reflecting changes of exploited grouper aggregations globally, as noted by fisher interviews, monitoring, or underwater surveys (N = 509). Kindred Grey. 2022. CC BY 4.0. Data from The Importance of Grouper and Threats to Their Future, by Yvonne Sadovy de Mitcheson and Min Liu Biology, in Ecology of Grouper. 2022. https://doi.org/10.1201/b20814.
Figure 13.6: Categories of all grouper species (N=167) according to the IUCN Red List (IUCN Red List Assessments, updated November 2018). Kindred Grey. 2022. CC BY 4.0. Data from The Importance of Grouper and Threats to Their Future, by Yvonne Sadovy de Mitcheson and Min Liu Biology, in Ecology of Grouper, 2022. https://doi.org/10.1201/b20814.
Figure 13.7: Large Nassau Grouper at The Pinnacle, Saba, Netherlands Antilles. Aquaimages, 2006. CC BY-SA 2.5. https://commons.wikimedia.org/wiki/File:3846_aquaimages.jpg.
Figure 13.8: Length-frequency distributions by sex for exploited and unexploited sites in Belize. Kindred Grey. 2022. CC BY 4.0. Data from NOAA, 2013. Public domain. https://www.fisheries.noaa.gov/resource/document/nassau-grouper-epinephelus-striatus-bloch-1792-biological-report.
Figure 13.9: Population estimates of Nassau Grouper at the spawning aggregation on Little Cayman Island from 2005 to 2018. Kindred Grey. 2022. CC BY 4.0. Data from Recovery of Critically Endangered Nassau Grouper (Epinephelus striatus) in the Cayman Islands Following Targeted Conservation Actions, by Waterhouse et. al., 2020. https://doi.org/10.1073/pnas.1917132117.
Figure 13.10: Figure 13.10: Historical postcard photo showing six Giant Sea Bass harvested by anglers. Florida Keys History Center, Monroe County Public Library, 2016. CC BY 2.0. https://flic.kr/p/PKAodm. Photo with correct identification can be found here https://www.metmuseum.org/art/collection/search/727682 or here https://www.islapedia.com/index.php?title=File:Meteor_Boat_Co_Bass_Catch©.jpg.
Figure 13.11: Conceptual diagram illustrating the biomass and population numbers of Goliath Grouper in south Florida. Kindred Grey. 2022. CC BY-SA 4.0. Adapted from Goliath Grouper Population (Florida), by Kris Beckert, Integration and Application Network (2008, CC BY-SA 4.0, https://ian.umces.edu/media-library/goliath-grouper-population-florida/). Includes Epinephelus itajara (Atlantic Goliath Grouper) 2 by Kim Kraeer, Lucy Van Essen-Fishman, Integration and Application Network, from https://ian.umces.edu/media-library/epinephelus-itajara-atlantic-goliath-grouper-2/ (CC BY-SA 4.0).
Figure 13.12: Yvonne Sadovy de Mitcheson, PhD. Used with permission from Yvonne Sadovy de Mitcheson. Photo by Alan Lai Kin Lun / Good Show Photography. CC BY-ND 4.0.
Text References
Aguilar-Perera, A. 2006. Disappearance of a Nassau Grouper spawning aggregation off the southern Mexico Caribbean coast. Marine Ecology Progress Series 327:289–296.
Aguilar-Perera, A. 2014. An obituary for a traditional aggregation site of Nassau Grouper in the Mexican Caribbean. Proceedings of the Gulf and Caribbean Fisheries Institute 66:384–388.
Aguilar-Perera, A., and W. Aguilar-Dávila. 1996. A spawning aggregation of Nassau Grouper Epinephelus striatus (Pisces: Serranidae) in the Mexican Caribbean. Environmental Biology of Fishes 45:351–361.
Aguilar-Perera, A., C. González-Salas, A. Tuz-Sulub, and H. Villegas-Hernández. 2009. Fishery of the Goliath Grouper, Epinephelus itajara (Teleostei: Epinephelidae) based on local ecological knowledge and fishery records in Yucatan, Mexico. International Journal of Tropical Biology 57:557–566.
Amorim, P., P. Sousa, M. Westmeyer, and G. M. Menezes. 2018. Generic Knowledge Indicator (GKI): a tool to evaluate the state of knowledge of fisheries applied to snapper and grouper. Marine Policy 89:40–49.
Aronson, R. B., J. F. Bruno, W. F. Precht, P. W. Glynn, C. D. Harvell, L. Kaufman, C. S. Rogers, E. A. Shinn, and J. F. Valentine. 2003. Causes of coral reef degradation. Science 302(5650):1502–1504.
Asch, R. G., and B. Erisman. 2018. Spawning aggregations act as a bottleneck influencing climate change impacts on a critically endangered reef fish. Diversity and Distributions 24: 1712−1728.
Belharet, M., A. Di Franco, A. Calò, L. Mari, J. Claudet, R. Casagrandi, M. Gatto, J. Lloret, C. Sève, P. Guidetti and P. Melià. 2020. Extending full protection inside existing marine protected areas, or reducing fishing effort outside, can reconcile conservation and fisheries goals. Journal of Applied Ecology 57:1948–1957.
Bender, M. G., G. R. Machado, P. J. A. Silva, S. R. Floeter, C. Monteiro-Netto, O. J. Luiz, and C. E. L. Ferreira. 2014. Local ecological knowledge and scientific data reveal overexploitation by multigear artisanal fisheries in the southwestern Atlantic. PLoS ONE 9(10):e110332. doi: 10.1371/journal.pone.0110332.
Bertoncini, Á., A. Aguilar-Perera, J. Barreiros, M. Craig, B. Ferreira, and C. Koenig. 2018. Epinephelus itajara. The IUCN Red List of Threatened Species 2018: e.T195409A46957794.
Bolden, S. K. 2000. Long-distance movement of a Nassau Grouper (Epinephelus striatus) to a spawning aggregation in the central Bahamas. Fishery Bulletin 98:642–645.
Bravo-Calderon, A., A. Saenz-Arroyo, S. Fulton, A. Espinoza-Tenorio, and E. Sosa-Cordero. 2021. Goliath Grouper Epinephelus itajara oral history, use, and conservation status in the Mexican Caribbean and Campeche Bank. Endangered Species Research 45:283–300.
Brulé, T., J. Montero-Muñoz, N. Morales-López, and A. Mena-Loria. 2015. Influence of circle hook size on catch rate and size of Red Grouper in shallow waters of the southern Gulf of Mexico. North American Journal of Fisheries Management 35:1196–1208.
Bshary, R., A. Hohner, K. Ait-el-Duoudi, and H. Fricke. 2006. Interspecific communicative and coordinated hunting between grouper and Giant Moray Eels in the Red Sea. PLoS Biology 4(12):e431.
Bueno, L. S., A. A. Bertoncini, C. C. Koenig, F. C. Coleman, M. O. Freitas, J. R. Leite, T. F. de Souza, and M. Hostim-Silva. 2016. Evidence for spawning aggregations of the endangered Atlantic Goliath Grouper Epinephelus itajara in southern Brazil. Journal of Fish Biology 89:876–889.
Bullock, L. H., M. D. Murphy, M. F. Godcharles, and M. E. Mitchell. 1992. Age, growth, and reproduction of jewfish Epinephelus itajara in the eastern Gulf of Mexico. Fishery Bulletin 90:243–249.
Bunce, M., L. D. Rodwell, R. Gibb, and L. Mee. 2008. Shifting baselines in fishers’ perceptions of island reef fishery degradation. Ocean and Coastal Management 51:285–302.
Carter, J., G. J. Marrow, and V. Pryor. 1994. Aspects of the ecology and reproduction of Nassau Grouper, Epinephelus striatus, off the coast of Belize, Central America. Proceedings of the 43rd Gulf and Caribbean Institute 43:65–111.
Cass-Calay, S. L. and T. W. Schmidt. 2009. Monitoring changes in the catch rates and abundance of juvenile Goliath Grouper using the ENP creel survey, 1973–2006. Endangered Species Research 7:183–193.
Cheung, W. W. L., Y. Sadovy de Mitcheson, M. T. Braynen, and L. G. Gittens. 2013. Are the last remaining Nassau Grouper Epinephelus striatus fisheries sustainable? Status quo in The Bahamas. Endangered Species Research 20:27–39.
Chiappone, M., R. Sluka and K. S. Sealey. 2000. Grouper (Pisces: Serranidae) in fished and protected areas of the Florida Keys, Bahamas and northern Caribbean. Marine Ecology Progress Series 198: 261–272.
Chollett, I., M. Priest, S. Fulton, and W. D. Heyman. 2020. Should we protect extirpated fish spawning aggregation sites? Biological Conservation 241:108395. https://doi.org/10.1016/j.biocon.2019.108395.
Chong-Montenegro, C., and H. K. Kindsvater. 2022. Demographic consequences of small-scale fisheries for two sex-changing groupers of the tropical Eastern Pacific. Frontiers in Ecology and Evolution 10:850006. https://doi.org/10.3389/fevo.2022.850006.
Claro, R., and K. C. Lindeman. 2003. Spawning aggregation sites of snapper and grouper species (Lutjanidae and Serranidae) on the insular shelf of Cuba. Gulf and Caribbean Research 14 (2):91–106.
Claro, R., Y. Sadovy de Mitcheson, K. C. Lindeman, and A. R. Garcia-Cagide. 2009. Historical analysis of Cuban commercial fishing effort and the effects of management interventions on important reef fishes from 1960–2005. Fisheries Research 99:7–16.
Coleman, F. C., C. C. Koenig, and L. A. Collins. 1996. Reproductive styles of shallow-water grouper (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. Environmental Biology of Fishes 47:129–141.
Coleman, F. C., C. C. Koenig, G. R. Huntsman, J. A. Musick, A. M. Eklund, J. C. McGovern, R.W. Chapman, G. R. Sedberry, and C. B. Grimes. 2000. American Fisheries Society position statement: long-lived reef fishes: the grouper-snapper complex. Fisheries 25(3):14–21.
Coleman, F. C,. K. C. Scanlon, and C. C. Koenig. 2011. Grouper on the edge: shelf edge spawning habitat in and around marine reserves of the northeastern Gulf of Mexico. Professional Geographer 63(4):1–19.
Colin, P. L. 1994. Preliminary investigations of reproductive activity of the jewfish, Epinephelus itajara (Pisces: Serranidae). Proceedings of the Gulf and Caribbean Fisheries Institute 43:138–147.
Colin, P. L. 1992. Reproduction of the Nassau grouper, Epinephelus striatus (Pisces: Serranidae), and its relationship to environmental conditions. Environmental Biology of Fishes 34:357–377.
Collins, D. 2022. Florida moves to officially lift 32-year ban on Goliath Grouper fishery. Outdoor Life, March 4. Available at: https://www.outdoorlife.com/fishing/florida-votes-to-officially-lift-32-year-ban-on-goliath-grouper/.
Colman, J. G. 1997. A review of the biology and ecology of the Whale Shark. Journal of Fish Biology 51:1219–1234.
Côté, I. M., J. A. Gill, T. A. Gardner, and A. R. Watkinson. 2005. Measuring coral reef decline through meta-analysis. Philosophical Transactions of the Royal Society B 360:385–395.
Craig, M. T., Y. J. Sadovy de Mitcheson, and P. C. Heemstra. 2011. Groupers of the world: a field and market guide. National Inquiry Services Center, Grahamstown, South Africa.
Dennis, L. P., G. Ashford, T. Q. Thai, V. V. In, N. H. Ninh, and A. Elizur. 2020. Hybrid grouper in Vietnamese aquaculture: production approaches and profitability of a promising new crop. Aquaculture 522:735108. https://doi.org/10.1016/j.aquaculture.2020.735108.
Domeier, M. L. 2012. Revisiting spawning aggregations: definitions and challenges. Pages 1–20 in Y. Sadovy de Mitcheson and P. L. Colin, editors, Reef fish spawning aggregations: biology, research and management, Springer, New York.
Dulvy, N. K., R. P. Freckleton, and N. V. C. Polunin. 2004. Coral reef cascades and the indirect effects of predator removal by exploitation. Ecology Letters 7:410–416.
Eggleston, D. B., J. J. Grover, and R. N. Lipcius. 1998. Ontogenetic diet shifts in Nassau Grouper: trophic linkages and predatory impact. Bulletin of Marine Science 63:111–126.
Erisman, B. E., L. G. Allen, J. T. Claisse, D. J. Pondella II, E. F. Miller, and J. H. Murray. 2011. The illusion of plenty: hyperstability masks collapses in two recreational fisheries that target fish spawning aggregations. Canadian Journal of Aquatic Sciences 68:1705–1716.
Erisman, B. E., W. D. Heyman, S. Fulton, and T. Rowell 2018. Fish spawning aggregations: a focal point of fisheries management and marine conservation in Mexico. Gulf of California Marine Program, La Jolla.
Erisman, B. E., W. Heyman, S. Kobara, T. Ezer, S. Pittman, O. Aburto-Oropeza, and R. S. Nemeth. 2017. Fish spawning aggregations: where well-placed management actions can yield big benefits for fisheries and conservation. Fish and Fisheries 18:128–144.
Erisman, B. E., C. McKinney-Lambert, and Y. Sadovy de Mitcheson. 2013. Sad farewell to C. Lavett-Smith’s iconic Nassau spawning aggregation site. Proceedings of the Gulf and Caribbean Fisheries Institute 66:421–422.
FAO. 2009. The state of world fisheries and aquaculture 2008. Food and Agricultural Organization, Fisheries Department, Rome.
Frías-Torres, S. 2006. Habitat use of juvenile Goliath Grouper Epinephelus itajara in the Florida Keys, USA. Endangered Species Research 2:1–6.
Frías-Torres, S. 2013. Should the critically endangered Goliath Grouper Epinephelus itajara be culled in Florida? Oryx 47(1): 88–95. doi:10.1017/S0030605312000361.
Fuiman, L. A., T. L. Connelly, S. K. Lowerre-Barbieri, and J. W. McClelland. 2015. Egg boons: central components of marine fatty acid food webs. Ecology 96:362–372.
Giglio, V. J., M. G. Bender, C. Zapelini, and C. E. L. Ferreira. 2017. The end of the line? Rapid depletion of a large-sized grouper through spearfishing in a subtropical marginal reef. Perspectives in Ecology and Conservation 15:115–118.
Giglio, V. J., A. A. Bertoncini, B. P. Ferreira, M. Hostim-Silva, and M. O. Freitas. 2014. Landings of Goliath Grouper, Epinephelus itajara, in Brazil: despite prohibited over ten years, fishing continues. Brazilian Journal of Nature Conservation 12:118–123.
Graham, R. T., K. L. Rhodes, and D. Castellanos. 2009. Characterization of the Goliath Grouper Epinephelus itajara fishery of southern Belize for conservation planning. Endangered Species Research 7:195−204.
Grossman, G. D. 2015. The jewfish and me. Medium, November 22. Available at: https://garydavidgrossman.medium.com/the-jewfish-and-me-99e34b6a6693#.5wl45gitf.
Gutiérrez, N. L. R. Hilborn, and O. Defeo. 2011. Leadership, social capital and incentives promote successful fisheries. Nature 470:386–389.
Harrington, J., B. Awad, K. Kingon, and A. Haskins. 2009. Goliath Grouper study: a survey analysis of dive shop and charter boat operators in Florida. Final Report. Florida Fish and Wildlife Conservation Commission. Available at: https://cefa.fsu.edu/sites/g/files/imported/storage/original/application/a379d337b1af201412c51763eb86b5f0.pdf.
Hazlett, B., and H. E. Winn. 1962. Sound producing mechanism of the Nassau Grouper, Epinephelus striatus. Copeia 2:447–449.
Hensel, E., J. E. Allgeier, and C. A. Layman. 2019. Effects of predator presence and habitat complexity on reef fish communities in The Bahamas. Marine Biology 166, 136. https://doi.org/10.1007/s00227-019-3568-3.
Heppell, S. S., S. A. Heppell, F. C. Coleman, and C. C. Koenig. 2006. Models to compare management options for a protogynous fish. Ecological Applications 16:238–249.
Heyman, W. D., L. M. Carr, and P. S. Lobel. 2010. Diver ecotourism and disturbance to reef fish spawning aggregations: it is better to be disturbed than to be dead. Marine Ecology Progress Series 419:201–210.
Heyman, W. D., R. T. Graham, B. Kjerfve, and R. E. Johannes. 2001. Whale Sharks Rhincodon typus aggregate to feed on fish spawn in Belize. Marine Ecology Progress Series 215:275–282.
Hostim-Silva, M., A. A. Bertoncini, M. Borgonha, J. R. Leite, M. O. Freitas, F. A. Daros, L. S. Bueno, A. P. C. Farro, and C. C. Koenig. 2017. The Atlantic Goliath Grouper: conservation strategies for a critically endangered species in Brazil. Pages 367–405 in M. R. Rossi-Santos, and C. W. Finkl, editors, Advances in marine vertebrate research in Latin America, Springer, New York.
Howlett, S. J., R. Stafford, M. Waller, S. Antha, and C. Mason-Parker. 2016. Linking protection with the distribution of grouper and habitat quality in Seychelles. Journal of Marine Biology 2016:7851425. DOI:10.1155/2016/7851425.
Khasanah, M., N. Nurdin, Y. Sadovy de Mitcheson, and J. Jompa. 2020 Management of the grouper export trade in Indonesia. Reviews in Fisheries Science & Aquaculture 28:1–15.
Kindsvater, H., J. Reynolds, Y. Sadovy de Mitcheson, and M. Mange. 2017. Selectivity matters: rules of thumb for management of plate-sized, sex-changing fish in the live reef food fish trade. Fish and Fisheries 18:821–836.
Knowlton, N, and J. B. Jackson. 2008. Shifting baselines, local impacts, and global change on coral reefs. PLoS Biology 6:e54.
Koenig, C. C., F. C. Coleman, A. M. Eklund, J. Schull, and J. Ueland. 2007. Mangrove as essential nursery habitat for Goliath Grouper (Epinephelus itajara). Bulletin of Marine Science 80:567–586.
Koenig, C. C., F. C. Coleman, and K. Kingon. 2011. Pattern of recovery of the Goliath Grouper Epinephelus itajara population in the southeastern US. Bulletin of Marine Science 87:891–911.
Koenig, C. C., F. C. Coleman, and C. R. Malinowski. 2020. Atlantic Goliath Grouper of Florida: to fish or not to fish. Fisheries 45(1):20–32.
Kough, A. S. B. C. Gutzler, J. G. Tuttle, N. Palma, L. C. Knowles, and L. Waterhouse. 2022. Anthropause shows differential influence of tourism and a no-take reserve on the abundance and size of two fished species. Aquatic Conservation: Marine and Freshwater Ecosytems 32:1693–1709.
Lara, M. R., J. Schull, D. L. Jones, and R. Allman. 2009. Early life history stages of Goliath Grouper Epinephelus itajara (Pisces: Epinephelidae) from Ten Thousand Islands, Florida. Endangered Species Research 7(3):221–228.
Lavett-Smith, C. 1972. A spawning aggregation of Nassau Grouper, Epinephelus striatus (Bloch). Transactions of the American Fisheries Society 101:257–261.
Luiz, O. J., R. M. Woods, E. M. P. Madin, and J. S. Madin. 2016. Predicting IUCN extinction risk categories for the world’s data deficient groupers (Teleostei: Epinephelidae). Conservation Letters 9(5):342–350.
Malinowski, C. R. 2019. High mercury concentrations in Atlantic Goliath Grouper: spatial analysis of a vulnerable species. Marine Pollution Bulletin 143:81–91.
Maljkovic, A., T. E. van Leeuwen, and S. N. Cove. 2008. Predation on the invasive Red Lionfish, Pterois volitans (Pisces: Scorpaenidae), by native groupers in The Bahamas. Coral Reefs 27(3):501. DOI:10.1007/s00338-008-0372-9.
Mann, D. A., J. V. Locascio, F. C. Coleman, and C. C. Koenig. 2009. Goliath Grouper (Epinephelus itajara) sound production and movement patterns on aggregation sites. Endangered Species Research 7:229–236.
McClenachan, L. 2009. Historical declines of Goliath Grouper populations in south Florida, USA. Endangered Species Research 7:175–181.
McKenzie, L. J., L. M. Nordland, B. L. Jones, L. C. Cullen-Unsworth, C. Roelfsema, and R. K. F. Unsworth. 2020. The global distribution of seagrass meadows. Environmental Research Letters 15(7):074041. DOI: 10.1088/1748-9326/ab7d06.
Mourier, J., J. Maynard, V. Parravicini, L. Ballesta, E. Clua, M. L. Domeier and S. Planes. 2016. Extreme inverted trophic pyramid of reef sharks supported by spawning groupers. Current Biology 26:2011–2016.
Mumby, P. J. 2006. Connectivity of reef fish between mangroves and coral reefs: algorithms for the design of marine reserves at seascape scales. Biological Conservation 128:215–222. https://doi.org/10.1016/j.biocon.2005.09.042.
Mumby, P. J., A. R. Harborne, and D. R. Brumbaugh. 2011. Grouper as a natural biocontrol of invasive lionfish. PLoS ONE 6:e21510.
Pauly, D., and D. Zeller. 2015. Sea around us concepts, design and data. Sea Around Us, Institute for the Oceans and Fisheries, University of British Columbia, Vancouver, Canada.
Pavlowich, T., and A. R. Kapuscinski. 2017. Understanding spearfishing in a coral reef fishery: fishers’ opportunities, constraints, and decision-making. PLoS ONE 12(7):e0181617.
Paxton, A. B., S. L. Harter, S. W. Ross, C. M. Schobernd, B. J. Runde, P. J. Rudershausen, K. H. Johnson, K. W. Shertzer, N. M. Bacheler, J. A. Buckel, G. T. Kellison, and J. C. Taylor. 2021. Four decades of reef observations illuminate deep-water grouper hotspots. Fish and Fisheries 22:749–761.
Philips, S., S. Sitaraman, C. M. Hong, and G. Yan. 2008. Inside the affluent space: a view from the top to anticipate the needs of the emerging affluent. World Association of Research Professionals, April. Available at: https://www.warc.com/fulltext/ESOMAR/88139.htm.
Pierre, S., S. Gaillard, and N. Prevot. 2008. Grouper aquaculture: Asian success and Mediterranean trials. Aquatic Conservation Marine and Freshwater Ecosystems 18(3):297–308.
Pinnegar, J. K., and G. H. Engelhard. 2008. The “shifting baseline” phenomenon: a global perspective. Reviews in Fish Biology and Fisheries 18:1–16.
Porch, C. E., A.-M. Eklund, and G. P. Scott. 2006. A catch-free stock assessment model with application to Goliath Grouper (Epinephelus itajara) off southern Florida. Fishery Bulletin 104:89–101.
Reddy, S. M. W., A. Wentz, O. Aburto-Oropeza, M. Maxey, S. Nagavarapu, S., and H. M. Leslie. 2013. Evidence of market-driven size-selective fishing and the mediating effects of biological and institutional factors. Ecological Applications 23:726–741.
Ribeiro, A. R., L. M. A. Damasio, and R. A. M. Silvano. 2021. Fishers’ ecological knowledge to support conservation of reef fish (groupers) in the tropical Atlantic. Ocean and Coastal Management 204:105543. https://doi.org/10.1016/j.ocecoaman.2021.105543.
Rimmer, M. A., and B. Glamuzina. 2019. A review of grouper (Family Serranidae: Subfamily Epinephelinae) aquaculture from a sustainability science perspective. Reviews in Aquaculture 11(1):58–87.
Robinson, J., N. A .J. Graham, J. E. Cinner, G. R. Almany, and P. Waldie. 2015. Fish and fisher behaviour influence the vulnerability of groupers (Epinephelidae) to fishing at a multispecies spawning aggregation site. Coral Reefs 34:371–382.
Rocklin, D., I. Rojo, M. Muntoni, D. Mateos-Molina, I. Bejarano, A. Caló, M. Russell, J. Garcia, F. C. Félix-Hackradt, C. W. Hackradt, and J. A. García-Charton. 2022. Fisheries regulation groupers’ management and conservation. Pages 118–165 in F. C. Félix-Hackradt, C. W. Hackradt, and J. A. García-Charton, editors, Biology and ecology of groupers, CRC Press, Boca Raton, FL.
Rojo, I., J. D. Anadón and J. A. García-Charton. 2021. Exceptionally high but still growing predatory reef fish biomass after 23 years of protection in a Marine Protected Area. PLoS ONE 16(2):e0246335.
Rowell, T. J., R. S. Nemeth, M. T. Schärer and R. S. Appeldoorn. 2015. Fish sound production and acoustic telemetry reveal behaviors and spatial patterns associated with spawning aggregations of two Caribbean groupers. Marine Ecology Progress Series 518:239–254.
Rudershausen, P. J., J. A. Buckel, and E. H. Williams. 2007. Discard composition and release fate in the snapper and grouper commercial hook-and-line fishery in North Carolina, USA. Fisheries Management and Ecology 14:103–113.
Russ, G. R., J. R. Rizzari, R. A. Abesamis, and A. C. Alcala. 2021. Coral cover a stronger driver of reef fish trophic biomass than fishing. Ecological Applications 31(1):e02224. https://doi.org/10.1002/eap.2224.
Russell, M. W., B. E. Luckhurst, and K. C. Lindeman. 2012. Management of spawning aggregations. Pages 371–404 in Y. Sadovy de Mitcheson and P. L. Colin, editors, Reef fish spawning aggregations: biology, research and management, Springer, Dordrecht.
Sadovy, Y. 1994. Grouper stocks of the western central Atlantic: the need for management and management needs. Proceedings of the 60th Gulf and Caribbean Institute 60:577–584.
Sadovy, Y., and W. L. Cheung. 2003. Near extinction of a highly fecund fish: the one that nearly got away. Fish and Fisheries 4:86–99.
Sadovy, Y., and M. Domeier. 2005. Are aggregation fisheries sustainable? Reef fish fisheries as a case study. Coral Reefs 24: 254–262.
Sadovy, Y., T. J. Donaldson, T. R. Graham, F. McGilvray, G. Muldoon, M. Phillips, and M. Rimmer. 2003. While stocks last: the live reef food fish trade. Asian Development Bank, Manila.
Sadovy, Y., and P. P. F. Lau. 2002. Prospects and problems for mariculture in Hong Kong associated with wild-caught seed and feed. Aquaculture Economics and Management 6:177– 190.
Sadovy, Y., and J. Pet. 1998. Wild collection of juveniles for grouper mariculture: just another capture fishery? Live Reef Fish Information Bulletin 4:36–39.
Sadovy de Mitcheson, Y. 2020. Island of hope for the threatened Nassau Grouper. Proceedings of the National Academy of Sciences 117:2243–2244.
Sadovy de Mitcheson, Y. 2019. The live reef food fish trade; undervalued, overfished and opportunities for change. International Coral Reef Initiative.
Sadovy de Mitcheson, Y. 2016. Mainstreaming fish spawning aggregations into fisheries management calls for precautionary approach. BioScience 66(4):295–306.
Sadovy de Mitcheson, Y., M. T. Craig, A. A . Bertoncini, K. E. Carpenter, W. W. L. Cheung, J. H. Choat, A. S. Cornish, S. T. Fennessy, B. P. Ferreira, P. C. Heemstra, M. Liu, R. F. Myers, D. A. Pollard, K. L. Rhodes, L. A. Rocha, B. C. Russell, M. A. Samoilys, and J. Sanciangco. 2013. Fishing groupers towards extinction: a global assessment of threats and extinction risks in a billion dollar fishery. Fish and Fisheries 14:119–136.
Sadovy de Mitcheson, Y., C. Linardich, J. P. Barreiros, G. M. Ralph, A. Aguilar-Perera, P. Afonso, B. E. Erisman, D. A. Pollard, S. T. Fennessy, A. A. Bertoncini, R. J. Nair, K. L. Rhodes, P. Francour, T. Brulé, M. A. Samoilys, B. P. Ferreira, and M. T.Craig. 2020. Valuable but vulnerable: over-fishing and under-management continue to threaten grouper so what now? Marine Policy 116:103909. https://doi.org/10.1016/j.marpol.2020.103909.
Sadovy de Mitcheson, Y., and M. Liu. 2022. The importance of grouper and threats to their future. Pages 191–230 in F. C. Félix-Hackradt, C. W. Hackradt, and J. A. García-Charton, editors, Biology and ecology of groupers, CRC Press, Boca Raton, FL.
Sadovy de Mitcheson, Y., and X. Yin. 2015. Cashing in on coral reefs: the implications of exporting reef fishes. Pages 166–179 in C. Mora, editor, Ecology of fishes on coral reefs, Cambridge University Press.
Sala, E. Ballesteros, and R. M. Starr. 2001. Rapid decline of Nassau Grouper spawning aggregations in Belize: fishery management and conservation needs. Fisheries 26(10):23–30.
Sambrook, K., A. S. Hoey, S. Andréfouët, G. S. Cumming, S. Duce, and M. C. Bonin. 2019. Beyond the reef: the widespread use of non-reef habitats by coral reef fishes. Fish and Fisheries 20:903–920.
Sherman, K. D., C. P. Dahlgren, J. R. Stevens, and C. R. Tyler. 2016. Integrating population biology into conservation management for endangered Nassau Grouper Epinephelus striatus. Marine Ecology Progress Series 554:263–280.
Shideler, G. S., D. W. Carter, C. Liese, and J. E. Serafy. 2015a. Lifting the Goliath Grouper ban: angler perspectives and willingness to pay. Fisheries Research 161:156–165.
Shideler, G. S., and B. Pierce. 2016. Recreational diver willingness to pay for Goliath Grouper encounters during the months of their spawning aggregation off eastern Florida, USA. Ocean and Coastal Management 129:36–43.
Shideler, G. S., S. R. Sagarese, W. J. Harford, J. Schull, and J. E. Serafy. 2015b. Assessing the suitability of mangrove habitats for juvenile Atlantic Goliath Grouper. Environmental Biology of Fishes 98:2067–2082.
Southwick, R., D. Maycock and M. Bouaziz. 2016. Recreational fisheries economic impact assessment manual and its application in two study cases in the Caribbean: Martinique and The Bahamas. FAO Fisheries and Aquaculture Circular No. 1128. Bridgetown, Barbados.
Starr, R. M., E. Sala, E. Ballesteros, and M. Zabala. 2007. Spatial dynamics of the Nassau Grouper, Epinephelus striatus, in a Caribbean atoll. Marine Ecology Progress Series 343:239–249.
Stock, B. C., S. A. Heppell, L. Waterhouse, I. C. Dove, C. V. Pattengill-Semmens, C. M. McCoy, P. G. Bush, G. Ebanks-Petrie, and B. X. Semmens. 2021. Pulse recruitment and recovery of Cayman Islands Nassau Grouper (Epinephelus striatus) spawning aggregations revealed by in situ length-frequency data. ICES Journal of Marine Science 78:277–292.
Stump, K., C. P. Dahlgren, K. D. Sherman, and C. R. Knapp. 2017. Nassau Grouper migration patterns during full moon suggest collapsed historic fish spawning aggregation and evidence of an undocumented aggregation. Bulletin of Marine Science 93:375–389.
To, A. W. L., and Y. J. Sadovy de Mitcheson. 2009. Shrinking baseline: the growth in juvenile fisheries, with the Hong Kong grouper fishery as a case study. Fish and Fisheries 10:396–407.
Tupper, M., and N. Sheriff. 2008. Capture-based aquaculture of groupers. Pages 217–253 in A. Lovatelli and P. F. Holthus, editors, Capture-based aquaculture: global overview, FAO Fisheries Technical Paper No. 508, Rome.
Ulrich, R. M., D. E. John, G. W. Barton, G. S. Hendrick, D. P. Fries, and J. H. Paul. 2015. A handheld sensor assay for the identification of grouper as a safeguard against seafood mislabeling fraud. Food Control 53:81–90.
Usseglio, P., A. M. Friedlander, H. Koike, J. Zimmerhackel, A. Schuhbauer, T. Eddy, and P. Salinas-de-León. 2016. So long and thanks for all the fish: overexploitation of the regionally endemic Galapagos Grouper Mycteroperca olfax (Jenyns 1840). PLoS ONE 11(10):e0165167.
Valiela, I., J. L. Bowen, and J. K. York. 2001. Mangrove forests: one of the world’s threatened major tropical environments. BioScience 51(10):807–815.
Veblen, T. 1912. The theory of the leisure class. Macmillan, London.
Waschkewitz, R., and P. Wirtz. 1990. Annual migration and return to the same site by an individual grouper, Epinephelus alexandrinus (Pisces, Serranidae). Journal of Fish Biology 36:781–782.
Waterhouse, L., S. A. Heppell, C. V. Pattengill-Semmens, C. McCoy, P. Bush, B. C. Johnson, and B. X. Semmens. 2020. Recovery of critically endangered Nassau Grouper (Epinephelus striatus) in the Cayman Islands following targeted conservation actions. Proceedings of the National Academy of Sciences 117(3):1587–1595.
Waycott, M., C. M. Duarte, T. J. Carruthers, R. J. Orth, W. C. Dennison, S. Olyarnik, A. Calladine, J. W. Fourqurean, K. L. Heck Jr., A. R. Hughes, G. A. Kendrick, W. J. Kenworthy, F. T. Short, and S. L. Williams. 2009. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proceedings of the National Academy of Sciences of the United States of America 106:12377–12381.
Wealth-X. 2018. World ultra wealth report. Available at: https://www.wealthx.com/report/world-ultra-wealth-report-2018/.
Wilcox, C. 2016. Fishing with cyanide. Hakai Magazine, June 30. Available at: https://hakaimagazine.com/news/fishing-cyanide/.
Traveling aimlessly from place to place
Having the female reproductive organs come to maturity before the male
An organism having both male and female sex organs or other sexual characteristics, either abnormally or (in the case of some organisms) as the natural condition
Regarding reproduction. occurring occasionally and at irregular intervals
Place or deal with close together for contrasting effect
Faithfulness to a reproductive partner
Relating to or concerned with the interaction of social and economic factors
Test for measuring content
Eliminated from existence in the wild
Use of a water body for production of coldwater or warmwater fish in a hatchery or rearing station
Test(s) for measuring content

