6 Congenital Heart Disease
Learning Objectives
- Describe the malformations of heart and/or major vascular structures in common congenital heart diseases.
- Determine the flow of blood through the heart, and systemic and pulmonary circulations in common congenital heart diseases.
In this chapter we will look at congenital heart diseases on a disease-by-disease basis. It might also be useful to review your heart embryology as it is highly relevant to the defects we will be looking at.
Atrial Septal Defect (ASD)
Embryology
The most common atrial septal defects arise from:
- Failure of the osteum secundum (most common),
- Excessive resorption of the septum primum, or
- Failure of septum primum to fuse with endocardial cushions (less common).
A patent foramen ovale (20 percent of the population) is not a true ASD as no tissue is missing and the remaining tissue acts as a one-way valve, so a PFO does not have the same pathophysiology as a true ASD. ASDs are common in infants with Down syndrome, as are VSDs.
Pathophysiology
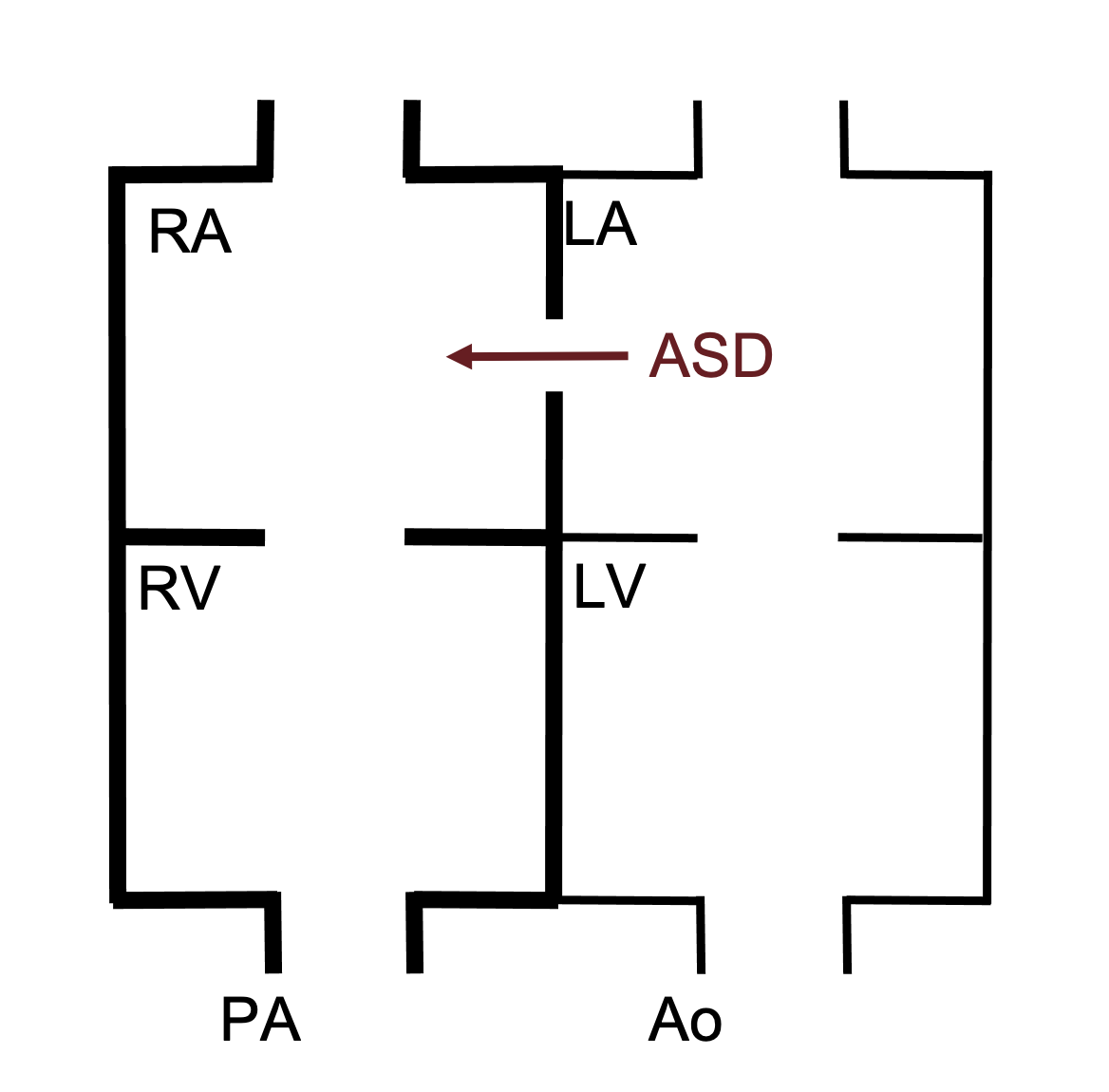
ASDs allow blood flow between the atria. As the pressure in the left atria is higher than that in the left, blood flows from left to right (figure 6.1). This causes volume overload of the right side of the heart. This excessive load may lead to right ventricular compliance being reduced as remodeling takes place. The reduced compliance can elevate right-side pressure and thereby reduce the left–right shunt.
Ventricular Septal Defect (VSD)
Embryology
Most ventricular septal defects arise from membraneous portion of the septum (70 percent), while others form in the muscular portion (20 percent); less frequently they occur near the aortic or AV valves.
Pathophysiology
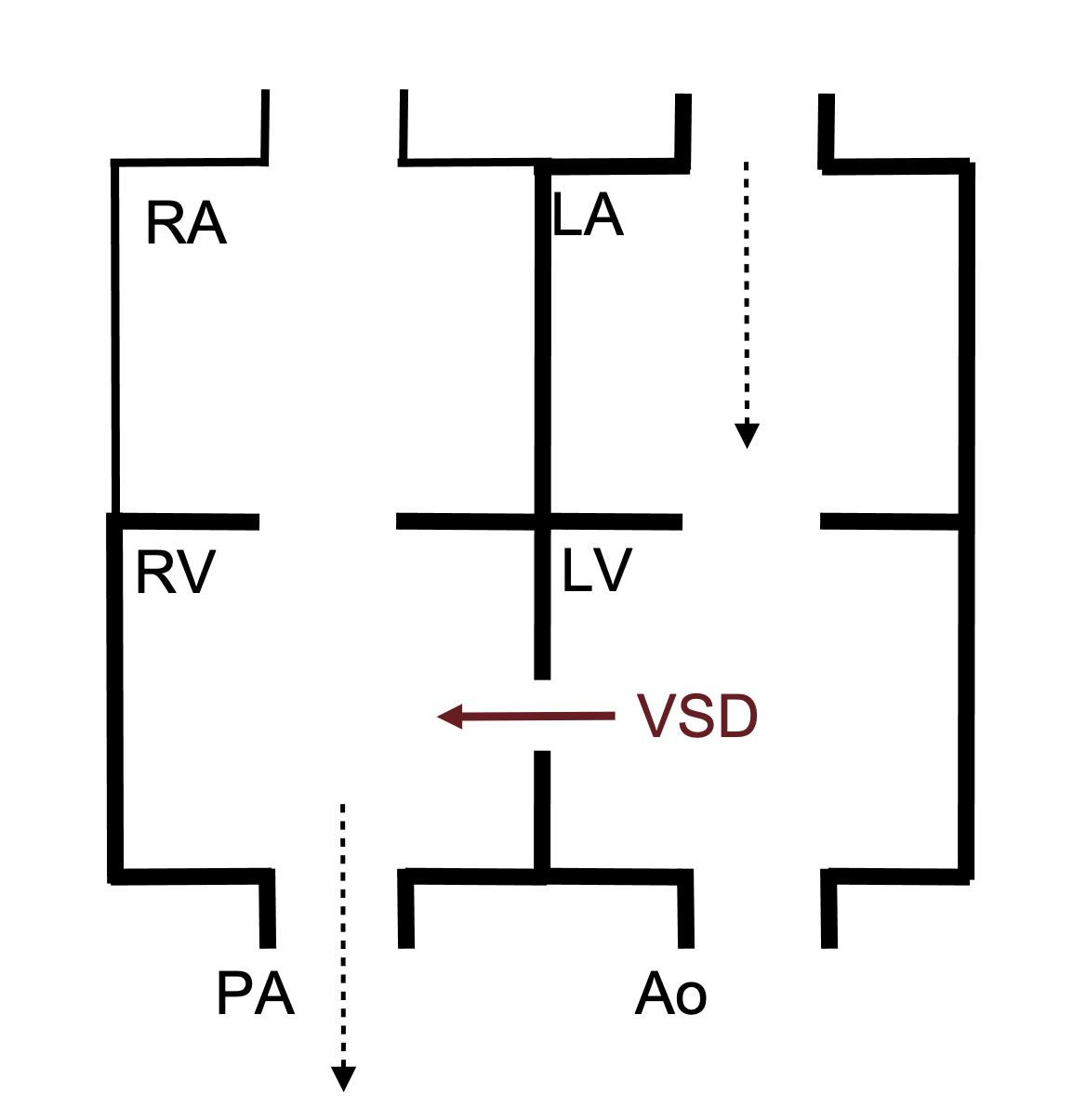
The manifestations of a VSD depend on the VSD size and the relative resistance of the pulmonary and systemic circulations—all of which will determine the direction of blood flow. During fetal development, the pulmonary and systemic circulations have equivalent resistances, so there may be very little shunting through the VSD, particularly if it is small. After birth, however, the resistance of the pulmonary system falls dramatically, so right ventricular pressure is lower and below left ventricular pressure (which still has to contend with systemic resistance)—consequently a left–right shunt is established. If this shunt is large (depending on the size of the defect), then blood returning from the pulmonary circulation to the left atrium can pass into the left ventricle, through the VSD into the right ventricle and head back into pulmonary circulation to start this loop again (figure 6.2).
When a large VSD is present, the recirculated blood causes volume overload of the right ventricle and the pulmonary circulation and subsequently both chambers of the left heart (figure 6.2). This can eventually cause chamber dilation and lead to heart failure. The extra volume load in the pulmonary circulation can also lead to early onset of pulmonary vascular disease.
Coarctation of the Aorta
Embryology
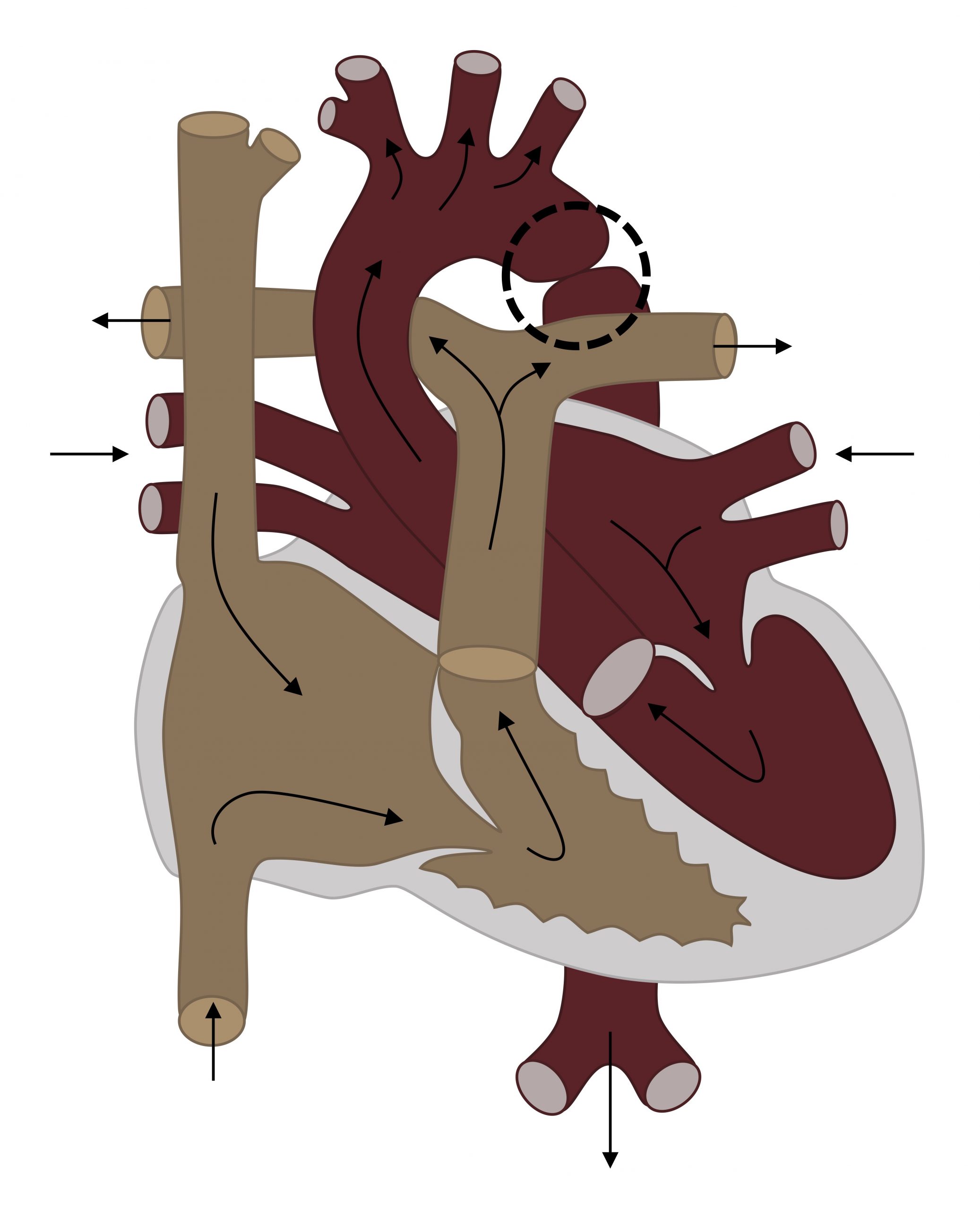
Coarctation of the aorta (figure 6.3) is a constriction of the aortic lumen, usually close to the ductus. The cause is unclear, but low flow through the left heart and aorta flow during development may cause the defect (no flow, no grow).
Pathophysiology
The diminished lumen causes increased afterload on the left ventricle. Vessels branching off the aorta before the coarctation can receive normal blood flow, so the head (carotid) and upper extremities (subclavian) are usually properly perfused whereas branching arteries after the coarctation may be underperfused. Consequently, differential cyanosis is a possible manifestation.
Tetralogy of Fallot (ToF)
Embryology
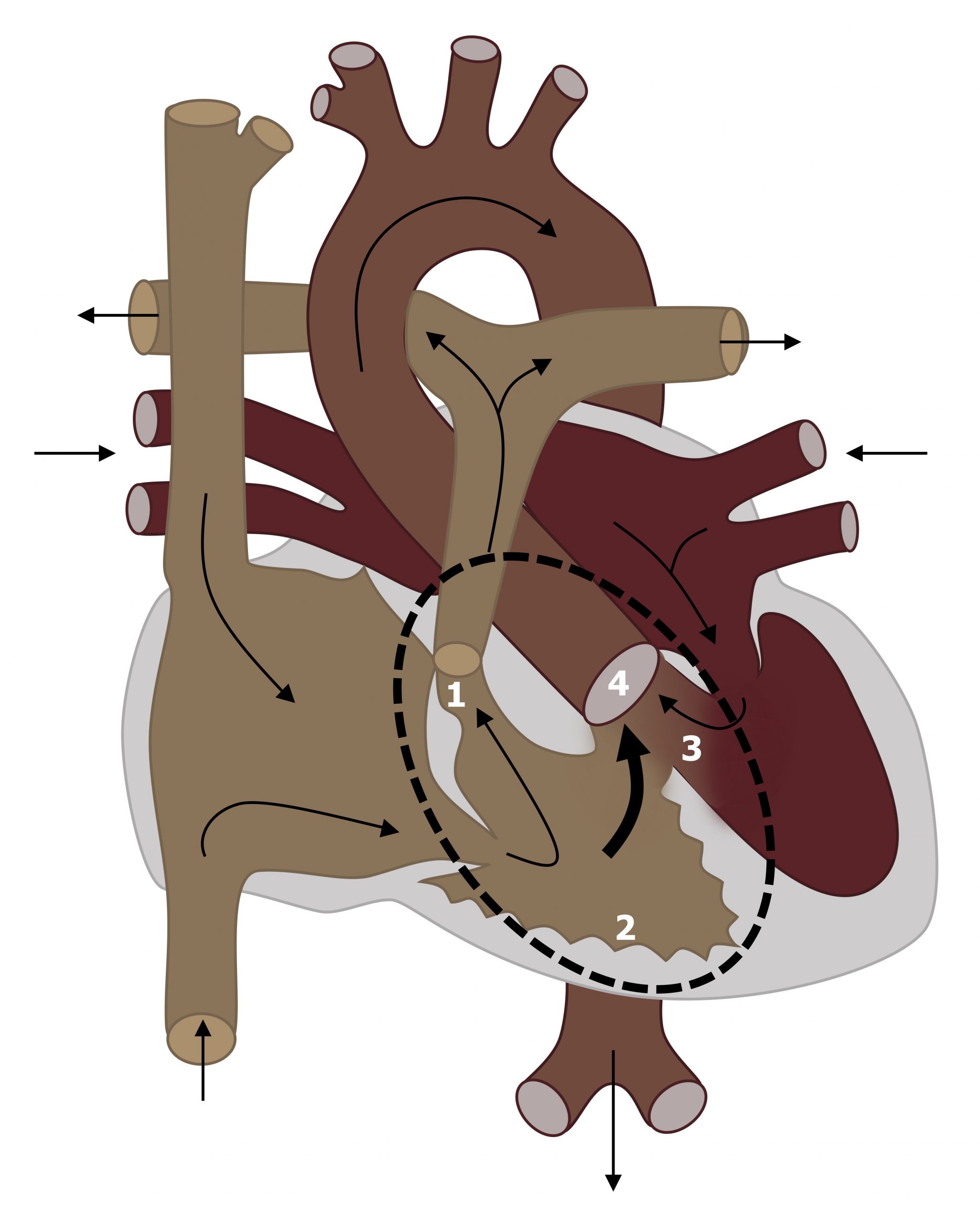
In Tetralogy of Fallot (ToF) the outflow tract (infundibular) portion of the interventricular septum is displaced. This single defect leads to four defects:
- Subvalvular pulmonic stenosis, because of the displaced infundibular septum (#1, figure 6.4),
- Right ventricular hypertrophy caused by the pulmonic stenosis (#2, figure 6.4),
- VSD—caused by malalignment of the interventricular septum (#3, figure 6.4), and
- Overriding aorta that receives blood from both ventricles (#4, figure 6.4).
Other defects can be associated with ToF, but the defects listed above lead this to be the most common form of cyanotic congenital heart disease.
Pathophysiology
The high resistance of the stenosed pulmonic valve (#1, figure 6.4) causes the blood in the right ventricle to exit through VSD (#3, figure 6.4) and enter the left ventricle forming a right-left shunt, bypassing the pulmonary circulation. Consequently blood with venous PO2 enters the systemic circulation and hypoxemia/cyanosis results. The degree of hypoxemia/cyanosis that occurs depends on the degree of pulmonic stenosis.
Transposition of the Great Arteries
Embryology
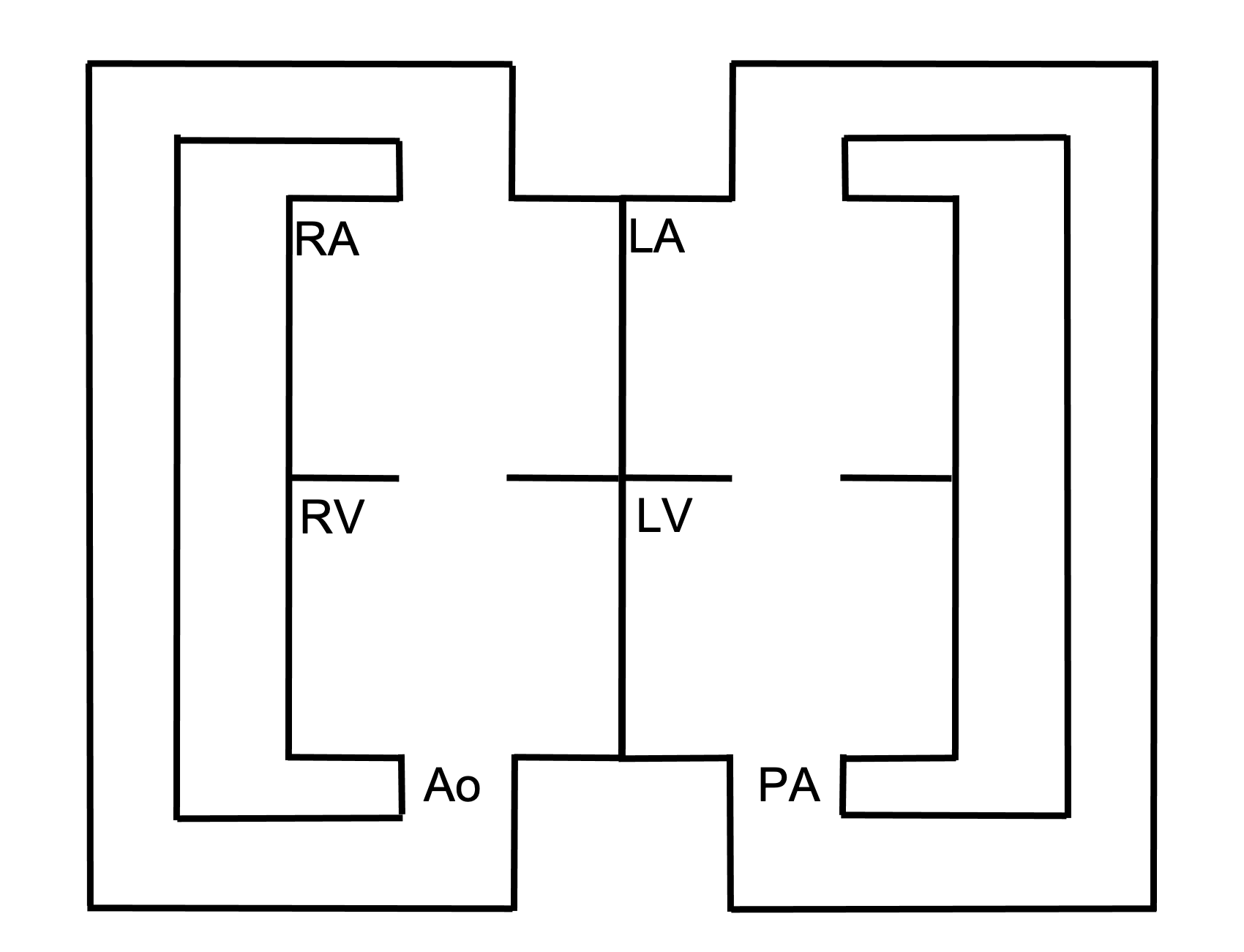
Although not completely understood, it is thought that failure of the aortic-pulmonary septum to spiral during development results in the great vessels coming off the wrong ventricles—the aorta exits the right, and the pulmonary artery exits the left. Other theories exist.
Pathophysiology
The placement of the pulmonary artery on the left means left ventricular blood is pumped up to pulmonary circulation, only to return to the left side of the heart via the pulmonary veins. Similarly, the aorta on the right forms a closed-system with the right ventricle pumping into the systemic circulation, only for it to return to the right atrium via the vena cava (see figure 6.5). So how is this compatible with life? In short, it is not. Embryonic development can continue because the two looped circulations can mix at the ductus arteriosus and foramen ovale of the fetal circulation. But after birth these shunts between the two circulations MUST be artificially maintained, or the patient must be “fortunate” enough to also have a VSD for mixing to take place.
Patent Ductus Arteriosus
Embryology
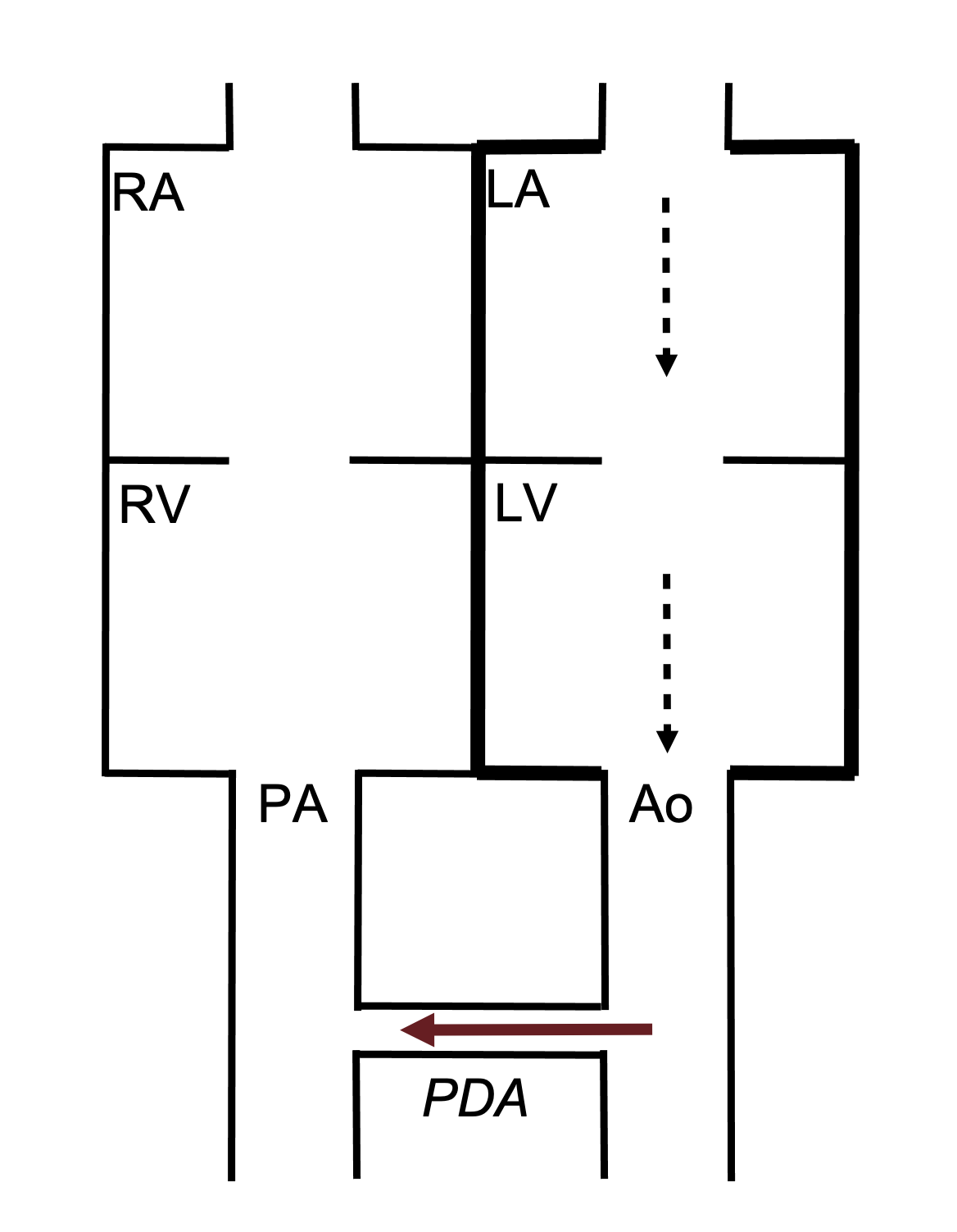
The ductus arteriosus is part of the fetal circulation allowing blood in the pulmonary artery to bypass the nonfunctional, high resistance lungs and instead traverse into the aorta and systemic circulation. The ductus should close at birth, and failure to do so leaves a patent ductus arteriosus (PDA).
Pathophysiology
In utero, the high resistance of the pulmonary circulation ensures that blood is diverted through the ductus arteriosis into the aorta. However, at birth there is a dramatic fall in the resistance of the pulmonary circulation as the lungs inflate. The pressure gradient across the ductus is consequently reversed (low on the pulmonary side, high on the systemic), so if the ductus remains open blood will flow from the aorta to the pulmonary artery (i.e., the opposite direction to fetal circulation) (figure 6.6).
The consequences of this are that a greater volume of blood reenters the pulmonary circulation, the left atria, and the left ventricle. Consequently the compartments of the left heart can eventually fail through volume overload. When the left heart fails, the shunt through the PDA can be reversed, and desaturated blood destined for the pulmonary circulation can end up passing through the PDA to the aorta instead—this reversal later in life is called Eisenmenger syndrome. In Eisenmenger’s the upper extremities receive uncontaminated, saturated blood, as their branching arteries are upstream of the desaturated blood entering the aorta at the PDA. Not so for the lower extremities whose arteries branch after the PDA and so receive low oxygen blood. Hence in Eisenmenger syndrome patients, only the feet are cyanosed.
Atrioventricular Canal
Embryology
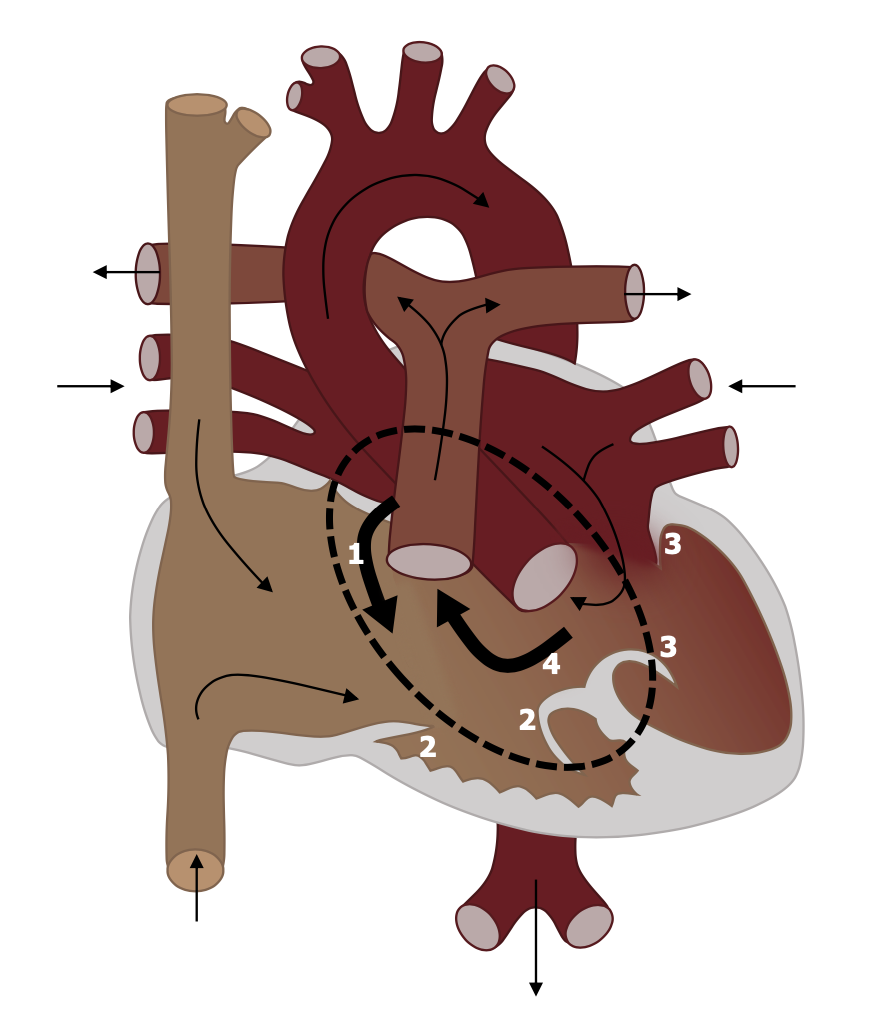
Complete AV canal defect is a result of complete failure of fusion between endocardial cushions. It is characterized by a primum atrial septal defect (#1, figure 6.7) that is contiguous with a ventricular septal defect (#4 in figure 6.7) and a malformed or common AV valve. Although several forms of this defect exist, this complete form is effectively a single chambered heart.
Pathophysiology
The malformed valves allow regurgitation, and the unrestricted interventricular communication allow a profound left–right shunt. This leads to volume overload in the pulmonary circulation, and heart failure will be produced if there is no correction. Pulmonary artery hypertension (PAH) and premature development of pulmonary vascular obstructive disease are other common outcomes.
Truncus Arteriosus
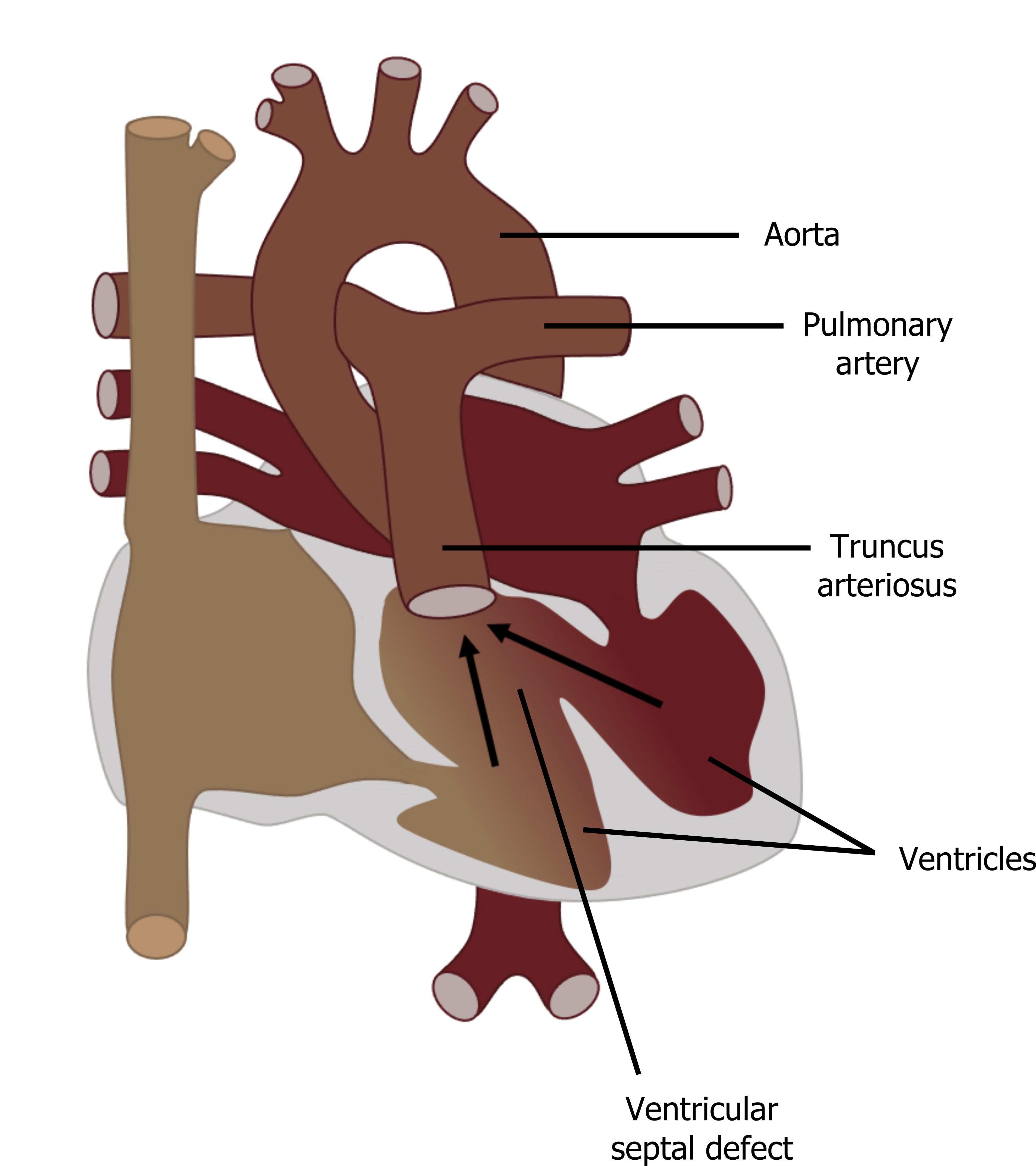
Embryology
Failed development of the truncoconal septum that normally leads to separation of the pulmonary artery and aorta leads to truncus arteriosus (TA). This condition leads to a single vessel with a single (often incompetent) valve positioned above the ventricular septum (see figure 6.8).
Pathophysiology
The underlying issues with TA are 1) mixing of blood from the left (saturated) and right heart (unsaturated), and 2) the common valve can allow regurgitation. In utero the high pulmonary vascular resistance means most blood exiting the heart goes through the aorta and cardiac output is rarely affected. At birth mild cyanosis can be produced by the mixing of blood from the left and right heart, but as pulmonary vascular resistance remains high in the first few days of life, cardiac output my be maintained. As pulmonary vascular resistance continues to fall in the first few weeks of life, a significant left–right shunt can become established as more left ventricular blood finds it “easier” to ascend up the pulmonary artery. Similar to a VSD, this leads to volume overload in the pulmonary circulation and eventually heart failure. The heart failure has a more rapid onset in TA than VSD if the common valve allows regurgitation. The regurgitation lowers end-diastolic ventricular volumes, so cardiac work to maintain cardiac output increases and promotes myocardial ischemia. Add to this the left–right shunt (as seen in VSD) and heart failure is more likely.
References, resources, and further reading
Text
Bhansali, Suneet, and Colin Phoon. Truncus Arteriosis. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK534774/, CC BY 4.0.
Cunningham, Jonathan W., and David W. Brown. “Congenital Heart Disease.” In Pathophysiology of Heart Disease: A Collaborative Project of Medical Students and Faculty, edited by Leonard S. Lilly, Chapter 16. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer Business, 2012.
Dakkak, Wael, and Tony I. Oliver. Ventricular Septal Defect. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470330/, CC BY 4.0.
Diaz-Frias, Josua, and Melissa Guillaume. Tetralogy of Fallot. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513288/, CC BY 4.0.
Gillam-Krakauer, Maria, and Kunal Mahajan. Patent Ductus Ateriosus. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK430758/, CC BY 4.0.
Law, Mark A., and Vijai S. Tivakaran. Coarctation of the Aorta. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK430913/, CC BY 4.0.
Menillo, Alexandra M., Lawrence S. Lee, and Anthony L. Pearson-Shaver. Atrial Septal Defect. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK535440/, CC BY 4.0.
Szymanski, Michael W., Sheila M. Moore, Stacy M. Kritzmire, and Amandeep Goyal. Transposition of the Great Arteries. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK430758/, CC BY 4.0.
Umapathi, Krishna Kishore, and Pradyumna Agasthi. Atrioventricular Canal Defects. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK557511/, CC BY 4.0.
Figures
Figure 6.1: Schematic of ASD showing left-right shunt. Thicker lines indicate presence of volume overload. Binks, Andrew. 2022. CC BY 4.0.
Figure 6.2: Schematic of VSD showing left-right shunt that can lead to volume overload in the RV, LA, LV and pulmonary circulation. Binks, Andrew. 2022. CC BY 4.0.
Figure 6.3: Coarctation of the aorta (circled). Grey, Kindred. 2022. CC BY 4.0. https://archive.org/details/6.3_20220113
Figure 6.4: Tetraology of fallot with (1) pulmonic stenosis, (2) RV hypertrophy, (3) VSD, and (4) overriding aorta. Grey, Kindred. 2022. CC BY 4.0. https://archive.org/details/6.4_20220113
Figure 6.5: Schematic of transposition of the great vessels (aorta off the right, pulmonary artery off the left) forming two separate, looped circulations. Binks, Andrew. 2022. CC BY 4.0.
Figure 6.6: Schematic of PDA with flow from aorta to pulmonary artery. Binks, Andrew. 2022. CC BY 4.0.
Figure 6.7: AV Canal with (1) ASD, (2&3) AV valve defects, and (4) VSD. Grey, Kindred. 2022. CC BY 4.0. https://archive.org/details/6.7_20220113
Figure 6.8: Truncus arteriosus. Grey, Kindred. 2022. CC BY 4.0. https://archive.org/details/6.8-copy

