4 Valvular Disease
Learning Objectives
- Describe the major causes of valvular incompetence or stenosis.
In basic terms, normal valves maintain normal direction of blood flow through the heart’s chambers. If they do not close properly they can allow backflow (regurgitation). If the valve does not fully open or is narrowed (stenosed), then the raised resistance impedes blood movement on its normal route and extra propulsive force must be applied by the myocardium. The clinical manifestations of cardiac valve disease vary depending on the valve involved, the form of dysfunction, and the severity and rate of onset of that dysfunction.
Abnormalities of valvular structure and/or function can either be congenital or acquired. Acquired valvular disease is by far the most common and is most prevalent in the elderly. The high blood flow and pressures that valves are exposed to make them particularly susceptible to other risk factors that promote valvular damage (see table 4.1). Congenital valvular defects arise from disrupted heart development, about 50 percent of which involve the valves. The impact of congenital defects has diminished with the advent of advanced detection techniques. What we will spend time on in this chapter is the main instigating factors and pathologies that result in acquired valvular defects.
| Risk factors |
|---|
| Age |
| Gender |
| Tobacco use |
| Hypercholesterolemia |
| Rheumatic heart disease |
| Hypertension |
| Type II diabetes |
Table 4.1: Risk factors for acquired valvular damage.
Pathophysiology of Valvular Disease
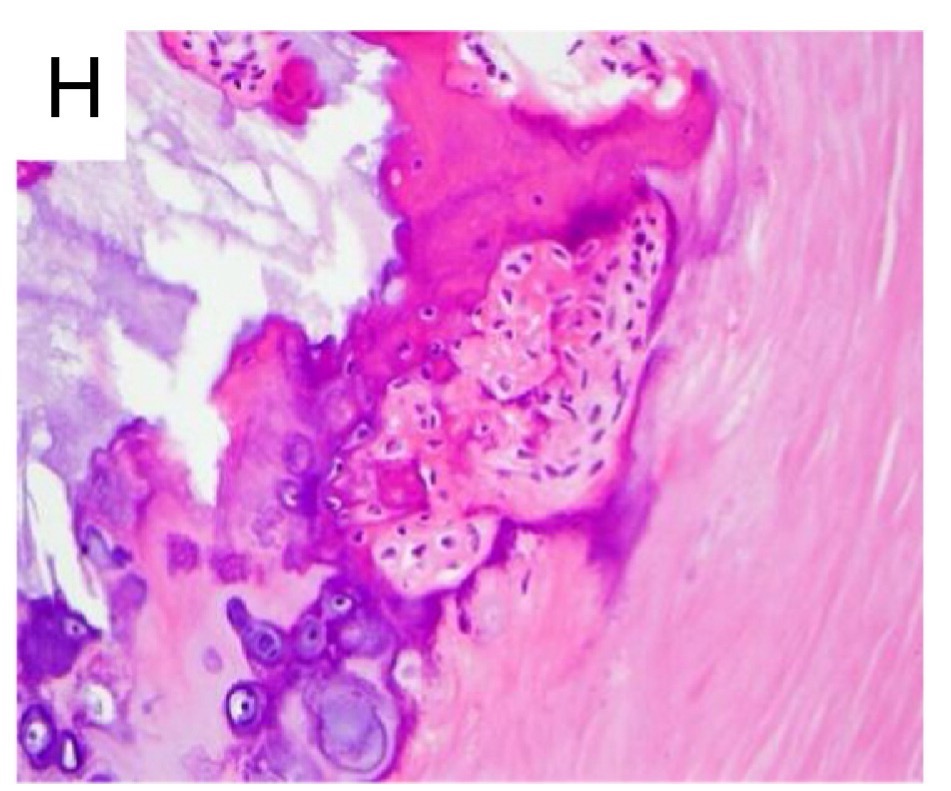
The constant stress of facing high flow and pressure over thirty to forty million cardiac contractions a year is not without its consequences, and the most common valvular disorder is calcification that comes with “wear-and-tear” and aging. The presence of other factors such as hyperlipidemia, hypertension, and inflammation accelerate this process and promote the deposition of hydroxyapatite (a form of calcium phosphate), and the valve structure contains cells that resemble osteoblasts (figure 4.1).
As they face the most pressure, the aortic and mitral valves are more prone to calcification. The most common pattern of calcification in the aortic valve is mounded masses within the cusps of the valve (see table 4.2) that eventually fuse and stop the valve from opening fully. Calcification in the mitral valve tends to start in the fibrous annulus, which does not impact valvular function to the same extent, but in exceptional cases can cause regurgitation or stenosis, or even arrhythmias as calcium deposits impinge on the atrioventricular conduction system (see table 4.2).
| Valve | Deposition of calcium | Gross path | Consequences |
|---|---|---|---|
| Aortic | Cusp | 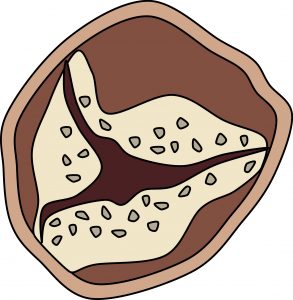 |
Stenosis |
| Mitral | Annulus | 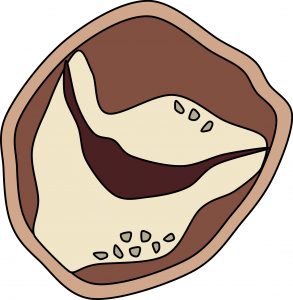 |
- Stenosis - Regurgitation - Arrhythmias |
Table 4.2: Location of calcium deposits on aortic and mitral valves and their pathological consequences.
Mitral Valve Prolapse (MVP)
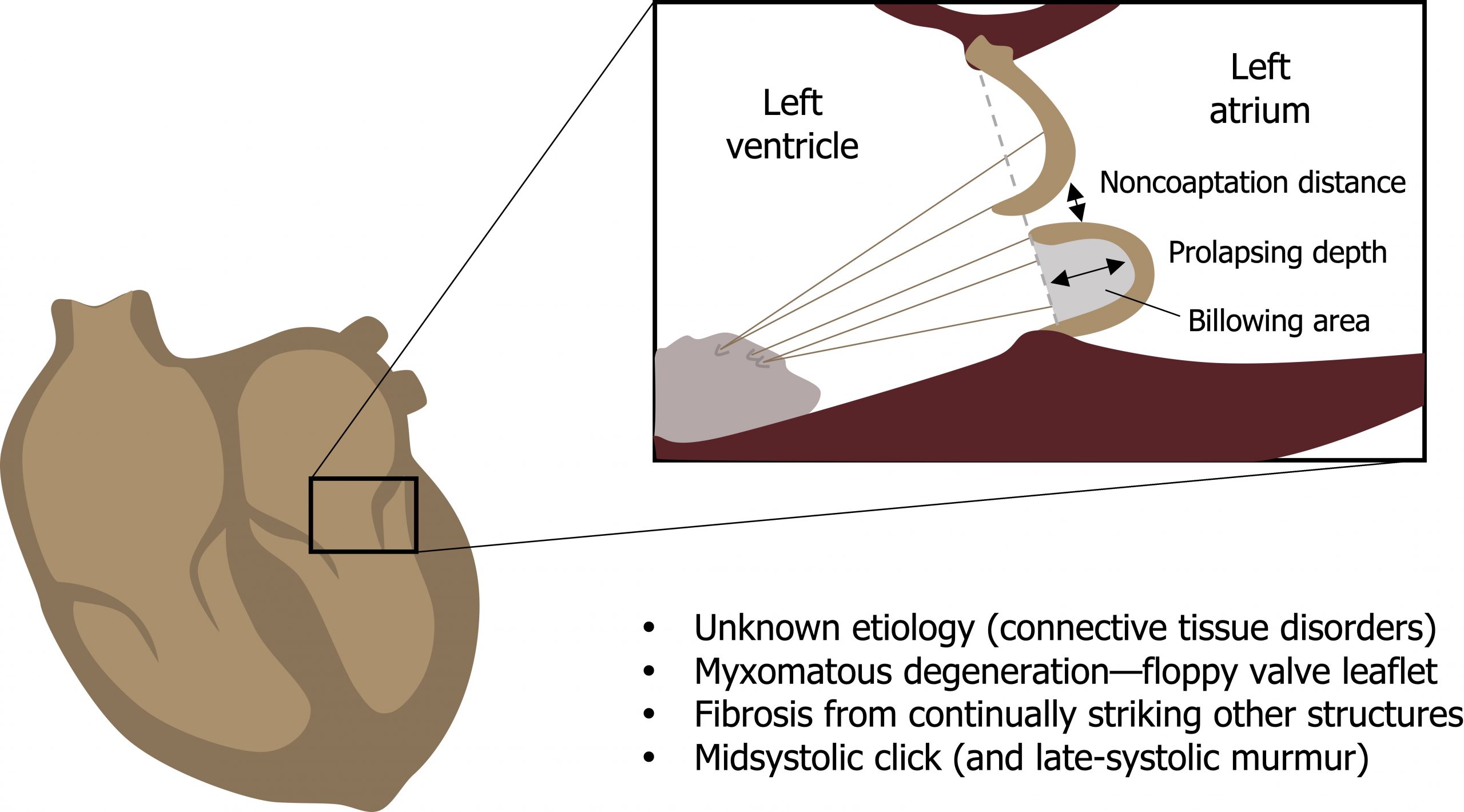
A prolapsed mitral valve is one where one or both leaflets have become floppy and capable of ballooning back into the left atrium during systole (figure 4.2). The condition is more common in women, affects 2–3 percent of adults in the United States, and can be a secondary effect of mitral valve regurgitation.
The causes of MVP are usually unidentified, but a few cases can be attributed to inherited connective tissue disorders such as Marfan syndrome. The prolapsed valve leaflet composition is enlarged and thickened with deposition of myxomatous material rich in proteoglycans, and a reduction in the structurally critical fibrosa layer where a higher prevalence of type III collagen (a more stretchy than structural form of collagen) is found.
The flapping valve structure can cause secondary fibrosis on the structures it strikes, such as the leaflet edges or the endocardium where the abnormally elongated cords rub. The agitation may also promote thrombus formation in the atrium.
The resultant floppy leaflet can be detected by a midsystolic click, and any associated incompetence may produce a late-systolic murmur (summary in figure 4.2). MVP is usually asymptomatic, but potential complications include:
- A propensity for endocardial infection,
- An increased risk of regurgitation and cord rupture,
- An increased stroke risk, and
- Higher incidence of arrhythmias.
Rheumatic Heart Disease
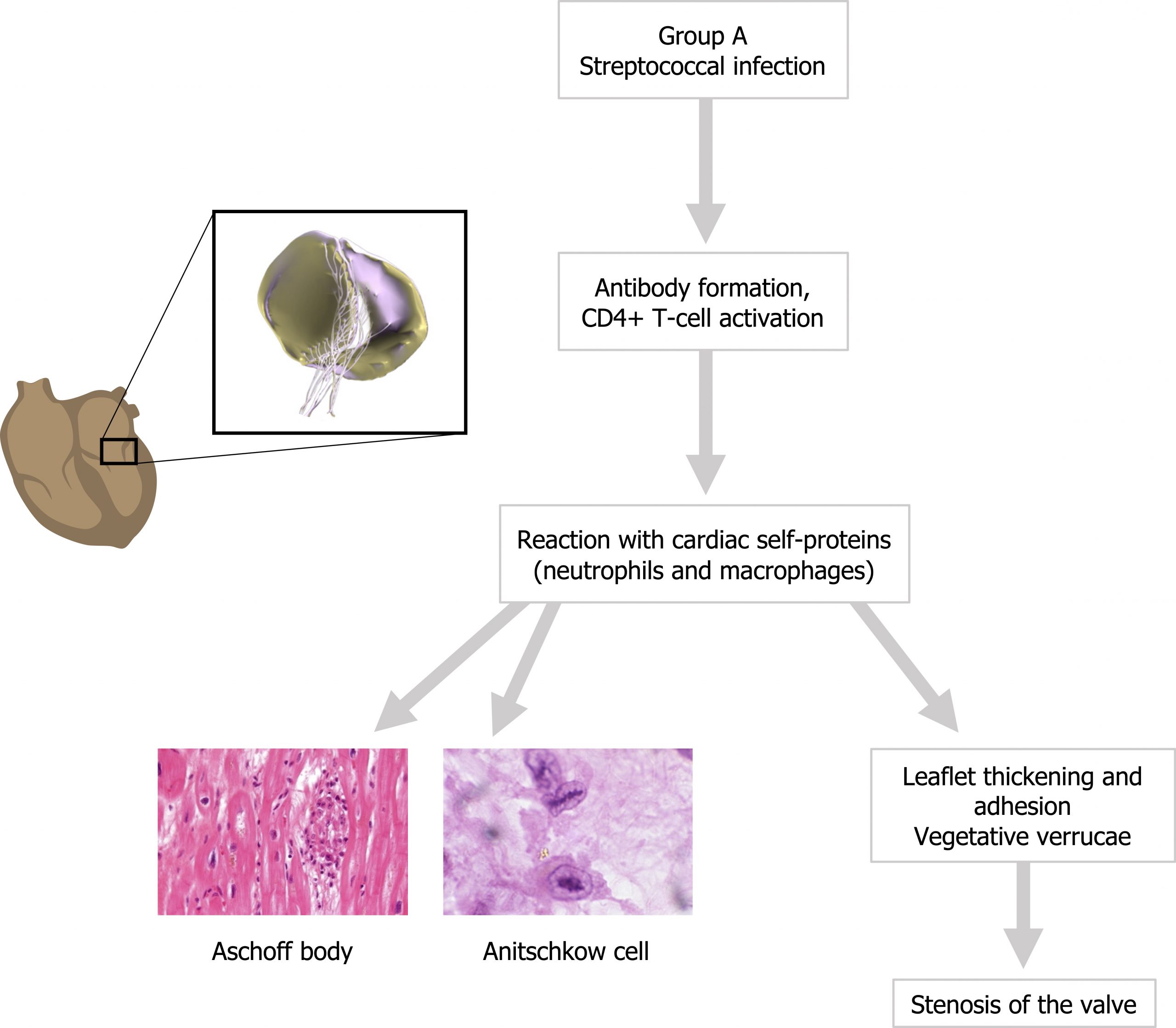
Rheumatic heart disease (RHD) is virtually the only cause of mitral valve stenosis. It arises after a group A streptococcal infection that often originates in the upper airway and leads to rheumatic fever (a multisystem, immune-mediated disease). The incidence in developed countries is relatively low because of rapid diagnosis and treatment of the instigating pharyngitis, but in poor, crowed, urban areas RHD remains an important health issue.
The acute results of rheumatic fever occur days to weeks after the streptococcal infection, and while the initial pharyngeal infection may have cleared and the test results have become negative, the antibodies to the streptococcal enzymes (Streptolysin O and DNase B) can still be detected. The initial cardiac effects include carditis, pericardial rubs, tachycardia, and arrhythmias. However, the chronic effects may arise years or even decades later.
The chronic effects involve an immune cross-reaction between the antibodies and CD4+ T-cells directed against the streptococcal M proteins and cardiac self-antigens. Antibody binding and T-cell activity toward the cardiac antigens activate complement and recruit neutrophils and macrophages toward the valve tissue. The damage they produce includes histologically distinct lesions called Aschoff bodies (figure 4.3), and plump activated macrophages called Anitschkow cells (or caterpillar cells) appear in the effected areas (figure 4.3). All layers of the myocardium can be effected, but the valves can show leaflet thickening and fusion as well as shortened, thickened cords. Vegetative verrucae are associated with the necrotic fibrinoid foci, making RHD one of the vegetative forms of valvular disease.
As the valve thickens it can become calcified as well, and the adhered leaflets produce a “fish-mouth” or “button hole” appearance that causes the valve to narrow. The damage is cumulative with the increased turbulence through a stenosed valve perpetuating the fibrotic process (see summary in figure 4.3).
Infective Endocarditis
Infective endocarditis (IE) is divided into acute and subacute forms, depending on the virulence of the causal pathogen. Acute IE is rapid in onset and involves highly destructive pathogens that cause necrosis and significant lesions that can lead to death in a matter of days. Subacute IE, alternatively, can deform the valves over weeks to months and generally involves a much less destructive pathogen.
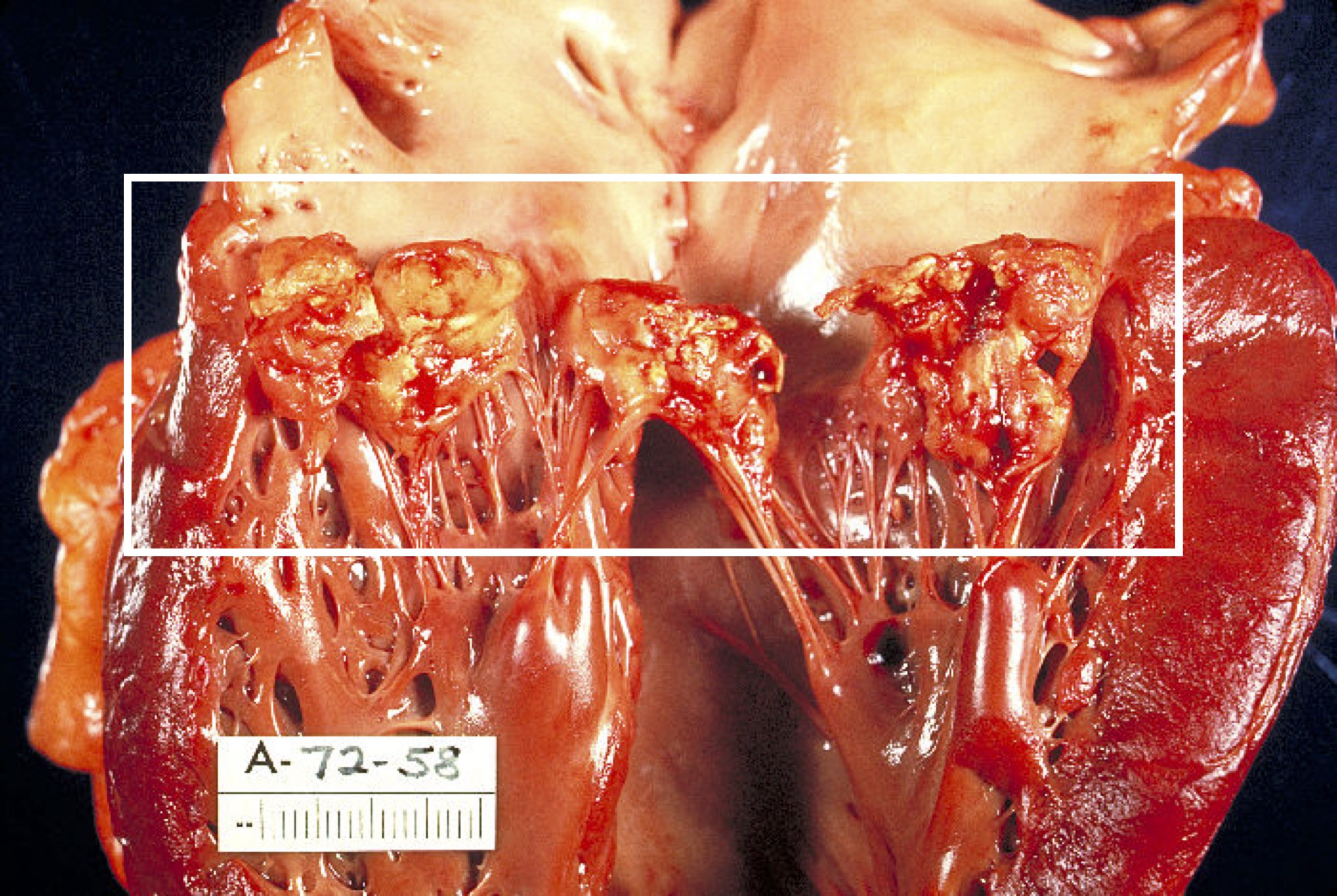
Acute cases tend to involve healthy individuals and are responsible for 20 to 30 percent of cases, whereas the less virulent pathogens that cause subacute IE tend to need a foothold and only affect previously damaged or deformed valves.
Most incidence of IE start with fever, but it can also manifest as nonspecific fatigue, weight loss, or flu-like symptoms in older adults. The infection leads to vegetations on the valve that are the hallmark of IE (figure 4.4). These lesions contain fibrin, inflammatory cells, and bacteria.
The risk is twofold as the vegetations can:
- Disrupt valve function and form abscesses into the underlying myocardium, and
- Embolize and carry the pathogens to a new septic infarcts or obstruct vasculature.
After a few weeks, complications arise that are the product of immune complex deposition or emboli. They can include glomerulonephritis as immune complexes become embedded in the glomerular basement membrane. Other later complications are now rare due to early detection and effective treatment but can include microthromboemboli that produce splinter or subungual lesions. Other hemorrhagic signs include Janeway lesions on the palms or soles, Osler nodes on the fingers, or Roth spots on the retina (figure 4.5).

Noninfective Vegetations
Some vegetations are sterile (i.e., occur in the absence of infection). There are two main examples of this—nonbacterial thrombotic endocarditis (NBTE) and the systemic lupus erythematosus (SLE).
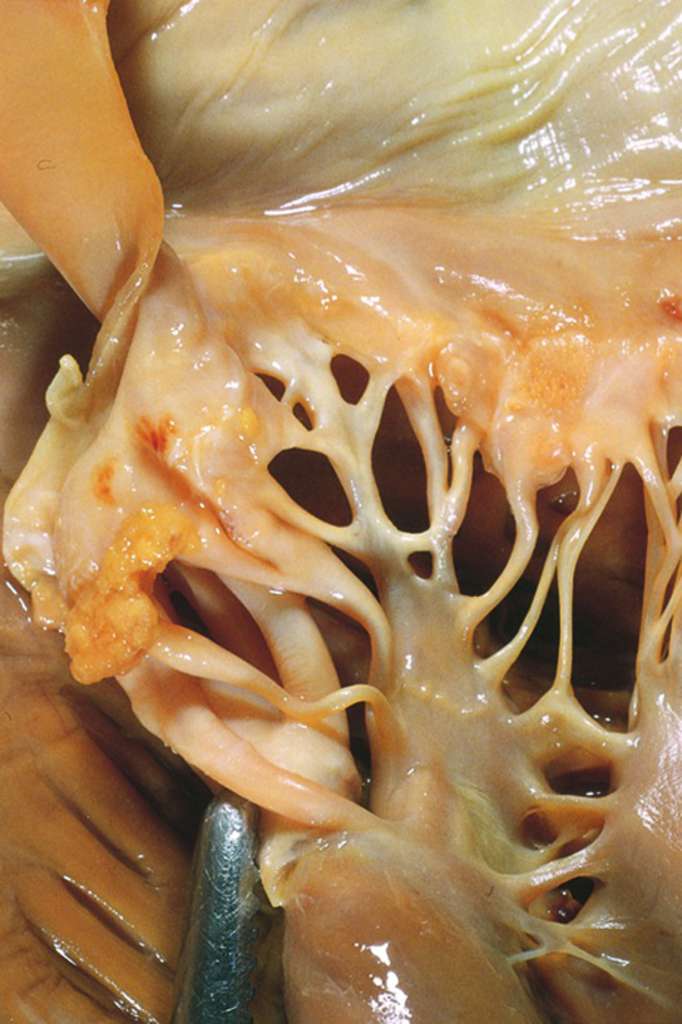
Often coinciding with emboli in other sites, NBTE occurs in states of hypercoagulability, such as in cancer or sepsis. The small thrombi (1–5 mm) bind to the valve leaflets (figure 4.6), but do not illicit an inflammatory response nor are they invasive. Often the local consequences are trivial, but they can be the source of emboli that lead to infarcts in the brain, heart, or elsewhere.
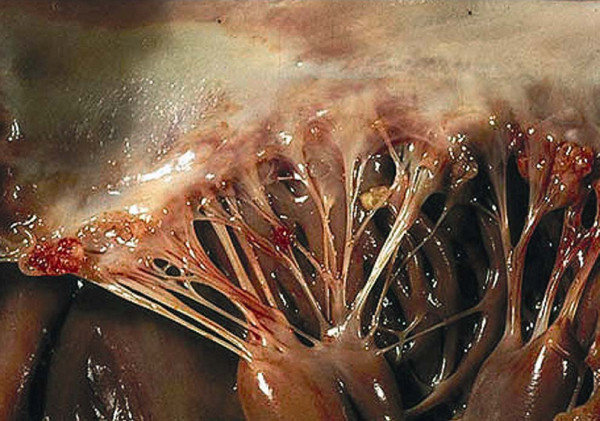
In SLE, the vegetations are again sterile and small (1–4 mm) with a pink, wart-like appearance that are composed of eosinophilic material, granular material, and cellular debris. They tend to adhere to the undersurfaces of the atrioventricular valves, the valvular endocardium, and the cords (figure 4.7). Unlike NBTE, the vegetations can instigate complement and Fc-bearing cells that cause intense valvulitis. The end product of this is referred to as Libman Sacks disease.
Carcinoid Heart Disease
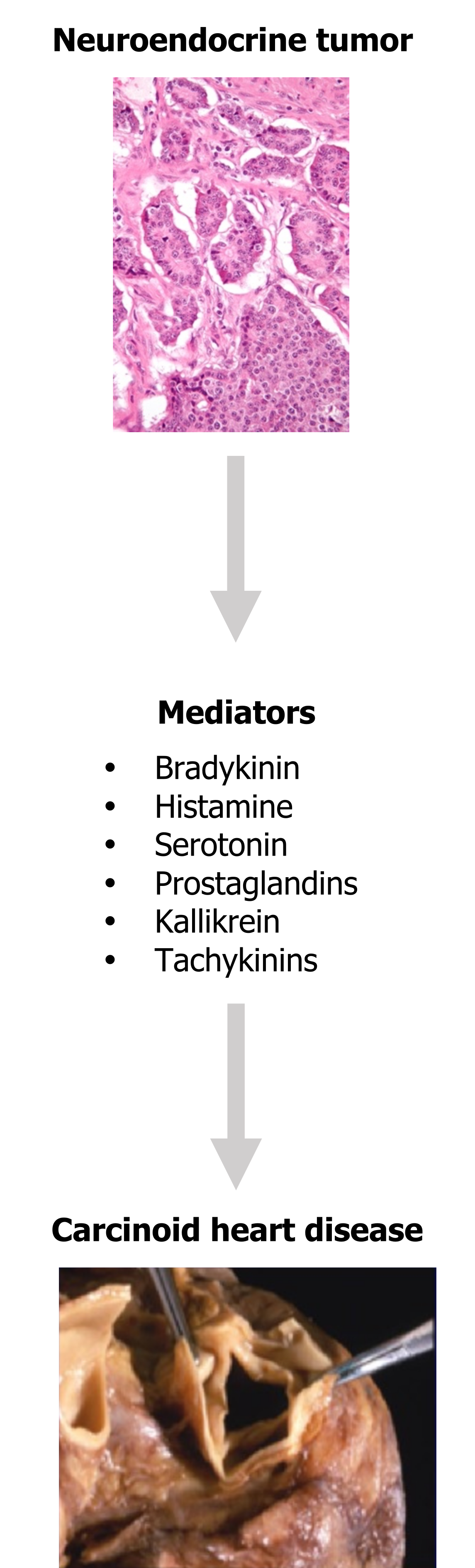
Lastly, carcinoid heart disease is the cardiac manifestation of carcinoid syndrome. Carcinoid tumors are neuroendocrine tumors that usually arise in the gastrointestinal tract or lungs, and they secrete a number of mediators (figure 4.8) that can give rise to carcinoid heart disease.
The liver normally metabolizes these circulating mediators, but when the metastatic burden overwhelms hepatic clearance, the right heart is exposed to their effects (the left heart is somewhat protected by the degradation performed by the pulmonary circulation).
Of all these released mediators, serotonin is the most likely candidate for causing cardiac effects, although the mechanism is not clear. Once established, carcinoid lesions are distinctive white intimal thickenings (figure 4.8) composed of smooth muscle cells and collagen embedded in a mucopolysaccharide matrix. The most common manifestations are tricuspid insufficiency and pulmonary stenosis.
References, resources, and further reading
Text
Dass, Clarissa, and Arun Kanmanthareddy. Rheumatic Heart Disease. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK538286/, CC BY 4.0.
Douedi, Steven, and Hani Douedi. Mitral Regurgitation. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK553135/, CC BY 4.0.
Lopez, Diana M., Patrick T. O’Gara, and Leonard S. Lilly. “Valvular Heart Disease.” In Pathophysiology of Heart Disease: A Collaborative Project of Medical Students and Faculty, 5e edited by Leonard S. Lilly, Chapter 8. Philadelphia: Lippincott Williams & Wilkins, a Wolters Kluwer Business, 2010.
Wenn, Peter, and Roman Zeltser. Aortic Valve Disease. Treasure Island, FL: StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK542205/, CC BY 4.0.
Figures
Figure 4.1: Histological view of ossification of valve tissue with osteoblast-like cells. Torre, Matthew. Figure 1, “Osseous and Chondromatous Metaplasia in Calcific Aortic Valve Stenosis.” Cardiovascular Pathology 25, no. 1 (January–February 2016): 18–24. From PubMed.gov, CC BY-NC-ND 4.0.
Table 4.1: Location of calcium deposits on aortic and mitral valves and their pathological consequences. Grey, Kindred. 2022. CC BY 4.0. https://archive.org/details/4.2a_20220113
Figure 4.2: Mitral valve prolapse. Grey, Kindred. 2022. CC BY 4.0. https://archive.org/details/4.3_20220113
Figure 4.3: Pathophysiology of rheumatic heart disease. Grey, Kindred. 2022. CC BY-SA 2.0. Added BruceBlaus. “Mitral Valve Prolapse.” 2017, from WikimediaCommons, CC BY-SA 4.0. Added Nephron. “Rheumatic Heart Disease – 3b – Very High Mag.” 2012, from WikimediaCommons, CC BY-SA 3.0. Added Uthman, Ed. “Anitschkow Myocytes in an Aschoff Body, Rheumatic Myocarditis.” 2007, from WikimediaCommons, CC BY-SA 2.0. https://archive.org/details/4.4_20220113
Figure 4.4: Vegetative lesions (in white box) associated with IE. Centers for Disease Control and Prevention. “Haemophilus parainfluenzae Endocarditis PHIL 851 Lores.” 1972, from WikimediaCommons, public domain.
Figure 4.5: Signs of IE include Janeway lesions (left), Osler nodes (middle) and Roth spots (right). Grey, Kindred. 2022. Added Community Eye Health. “Roth Spots and Retinal Haemorrhage.” 1998, from Flickr, CC BY-NC 2.0. Added Galindo, Roberto J. “Osler Nodules Hand.” 2010, from WikimediaCommons, CC BY-SA 4.0. Added Warfieldian. “Janeway Lesion.” 2015, from WikimediaCommons, CC BY-SA 4.0. https://archive.org/details/4.6_20220113
Figure 4.6: NBTE with small thrombi binding to valve leaflets (arrowed). Mohsin, Sara. “Libman-Sacks Endocarditis.” 2020, from WikiDoc, CC BY SA 3.0.
Figure 4.7: Small “wart-like vegetations” in the cords of a valve. Bouma, Wobbe, Iwan C. C. van der Horst, Theo J. Klinkenberg, and Inez J. Wijdh-den Hamer. “Mitral Valve Surgery for Mitral Regurgitation Caused by Libman-Sacks Endocarditis: A Report of Four Cases and a Systematic Review of the Literature.” Journal of Cardiothoracic Surgery 5, no. 1 (March 2010): 13. DOI: 10.1186/1749-8090-5-13. Reproduced in JCS with permission from Dr. S. Gonzalez. Copyright 2009, department of Pathology, Pontifical Catholic University of Chile, Santiago, Chile. Used under fair use.
Figure 4.8: Release of inflammatory mediators from neuroendocrine tumors leading to carcinoid heart disease. Grey, Kindred. 2022. Added Feingold, K. R., B. Anawalt, A. Boyce, et al., eds. Figure 24, Carcinoid Tumors. South Dartmouth, MA: MDText.com, Inc., 2000–. From NCBI, CC BY-NC-ND 2.0. Used under fair use. Added Nephron. “Small Intestine Neuroendocrine Tumour High Mag.” 2009, from WikimediaCommons, CC BY-SA 3.0.

