3 Systemic Infections of the Skin
3.1 Introduction to the Anatomy and Normal Microbiota of the Skin and Eyes
Human skin is an important part of the innate immune system. In addition to serving a wide range of other functions, the skin serves as an important barrier to microbial invasion. Not only is it a physical barrier to penetration of deeper tissues by potential pathogens, but it also provides an inhospitable environment for the growth of many pathogens. In this section, we will provide a brief overview of the anatomy and normal microbiota of the skin and eyes, along with general symptoms associated with skin and eye infections.
Layers of the Skin
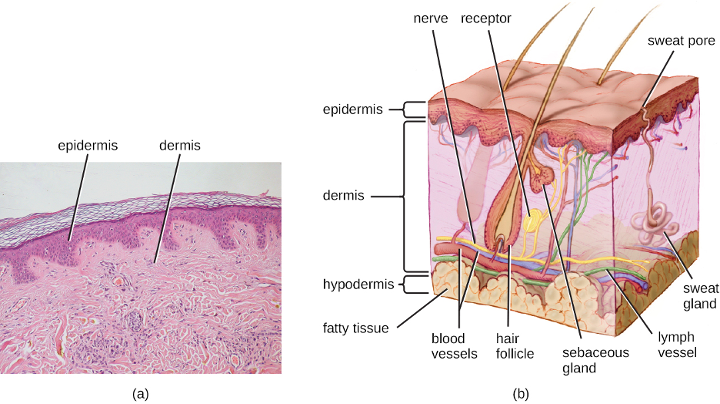
Human skin is made up of several layers and sublayers. The two main layers are the epidermis and the dermis. These layers cover a third layer of tissue called the hypodermis, which consists of fibrous and adipose connective tissue (figure 3.1).
The epidermis is the outermost layer of the skin and its thickness varies based on location. Areas such as the soles of the feet and palms of the hands display a thick epidermis to protect against mechanical forces, while the epidermis of areas such as the face are much thinner. The exterior surface of the epidermis, called the stratum corneum, primarily consists of dead skin cells. This layer of dead cells limits direct contact between the outside world and live cells. The stratum corneum is rich in keratin, a tough, fibrous scleroprotein that is a major structural intermediate filament of epithelial cells and is also found in hair and nails. Keratin helps make the outer surface of the skin relatively tough and waterproof. It also helps to keep the surface of the skin dry, which reduces microbial growth. However, some microbes are still able to live on the surface of the skin, and some of these can be shed with dead skin cells in the process of desquamation, which is the shedding and peeling of skin that occurs as a normal process but that may be accelerated when infection is present.
Beneath the epidermis lies a thicker skin layer called the dermis. The dermis contains connective tissue and embedded structures such as blood vessels, nerves, and muscles. Structures called hair follicles (from which hair grows) are located within the dermis, even though much of their structure consists of epidermal tissue. Indeed, the other epithelial layer of the hair follicle is actually an invagination of the overlying squamous epithelium. The dermis also contains the two major types of glands found in human skin: sweat (apocrine)glands (tubular glands that produce sweat) and sebaceous glands (which are associated with hair follicles and produce sebum, a lipid-rich substance containing proteins and minerals).
Perspiration (sweat) provides some moisture to the epidermis, which can increase the potential for microbial growth. For this reason, more microbes are found on the regions of the skin that produce the most sweat, such as the skin of the underarms and groin. However, in addition to water, sweat also contains substances that inhibit microbial growth, such as salts, lysozyme, and antimicrobial peptides. Sebum also serves to protect the skin and reduce water loss. Although some of the lipids and fatty acids in sebum inhibit microbial growth, sebum contains compounds that provide nutrition for certain microbes.
Normal Microbiota of the Skin
The skin is home to a wide variety of normal microbiota, consisting of commensal organisms that derive nutrition from skin cells and secretions such as sweat and sebum. The normal microbiota of skin tends to inhibit transient-microbe colonization by producing antimicrobial substances and outcompeting other microbes that land on the surface of the skin. This helps to protect the skin from pathogenic infection.
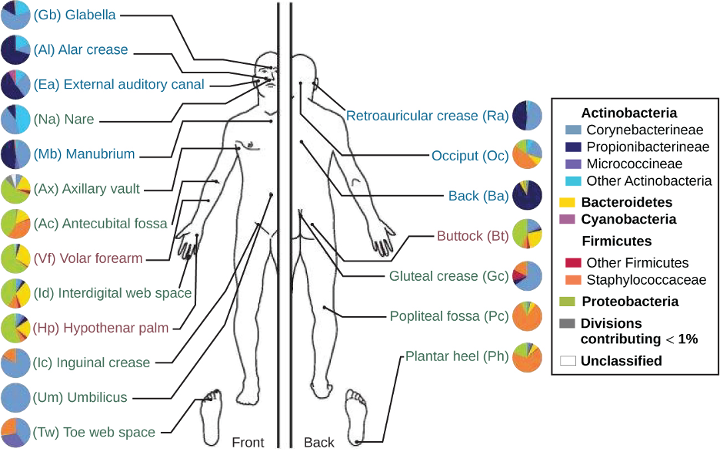
The skin’s properties differ from one region of the body to another, as does the composition of the skin’s microbiota. The availability of nutrients and moisture partly dictates which microorganisms will thrive in a particular region of the skin. Relatively moist skin, such as that of the nares (nostrils) and underarms, has a much different microbiota than the dryer skin on the arms, legs, hands, and top of the feet. Some areas of the skin have higher densities of sebaceous glands. These sebum-rich areas, which include the back, the folds at the side of the nose, and the back of the neck, harbor distinct microbial communities that are less diverse than those found on other parts of the body.
Different types of bacteria dominate the dry, moist, and sebum-rich regions of the skin. The most abundant microbes typically found in the dry and sebaceous regions are Betaproteobacteria and Propionibacteria, respectively. In the moist regions, Corynebacterium and Staphylococcus are most commonly found (figure 3.2). Viruses and fungi are also found on the skin; Malassezia is the most common type of fungus found within the normal microbiota. The role and populations of viruses in the microbiota, known as viromes, are still not well understood, and there are limitations to the techniques used to identify them. However, Circoviridae, Papillomaviridae, and Polyomaviridae appear to be the most common residents in the healthy skin virome.[1][2][3]
Infections of the Skin
While the microbiota of the skin can play a protective role, it can also cause harm in certain cases. Often, an opportunistic pathogen residing in the skin microbiota of one individual may be transmitted to another individual more susceptible to an infection. For example, methicillin-resistant Staphylococcus aureus (MRSA) can often take up residence in the nares of health care workers and hospital patients; though harmless on intact, healthy skin, MRSA can cause infections if introduced into other parts of the body, as might occur during surgery or via a post-surgical incision or wound. This is one reason why clean surgical sites are essential.
Injury or damage to the skin can allow microbes to enter deeper tissues, where nutrients are more abundant and the environment is more conducive to bacterial growth. Wound infections are common after a puncture or laceration that damages the physical barrier of the skin. Microbes may infect structures in the dermis, such as hair follicles and glands, causing a localized infection, or they may reach the bloodstream, which can lead to a systemic infection.
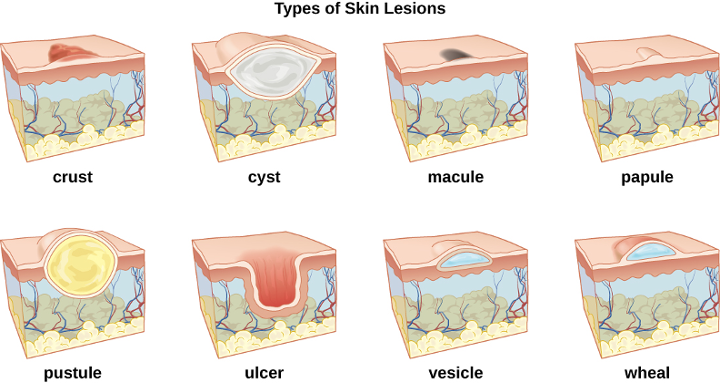
In some cases, infectious microbes can cause a variety of rashes or lesions that differ in their physical characteristics. These rashes can be the result of inflammation reactions or direct responses to toxins produced by the microbes. Table 3.1 lists some of the medical terminology used to describe skin lesions and rashes based on their characteristics and the most common skin infections are summarized in table 3.3. Figure 3.3 and figure 3.4 illustrate some of the various types of skin lesions. It is important to note that many different diseases can lead to skin conditions of very similar appearance; generally, the terms used in the table are not exclusive to a particular type of infection or disease.
| Term | Definition |
|---|---|
| abscess | localized collection of pus, often with an inflamed fibrous outer capsule |
| bulla (pl., bullae) | fluid-filled blister no more than 5 mm in diameter |
| carbuncle | deep, pus-filled abscess generally formed from multiple furuncles |
| crust | dried fluids from a lesion on the surface of the skin |
| cyst | encapsulated sac filled with fluid, semi-solid matter, or gas, typically located just below the upper layers of skin |
| folliculitis | a localized rash due to inflammation of hair follicles |
| furuncle (boil) | pus-filled abscess due to infection of a hair follicle |
| macules | smooth spots of discoloration on the skin |
| papules | small raised bumps on the skin |
| pseudocyst | lesion that resembles a cyst but is not lined by a defined epithelial capsule |
| purulent | pus-producing; suppurative |
| pustules | fluid- or pus-filled bumps on the skin |
| pyoderma | any suppurative (pus-producing) infection of the skin |
| suppurative | producing pus; purulent |
| ulcer | break in the skin with complete loss of the epidermal barrier; open sore |
| vesicle | small, fluid-filled lesion |
| wheal | swollen, inflamed skin that itches or burns, such as from an insect bite |
Table 3.1: Some medical terms associated with skin lesions and rashes
Anatomy and Microbiota of the Eye
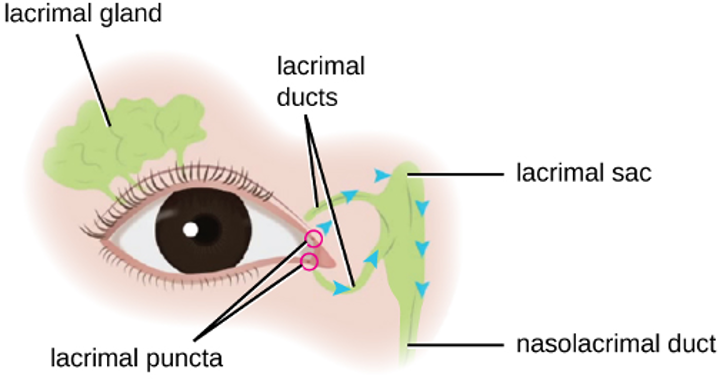
Although the eye and skin have distinct anatomy, they are both in direct contact with the external environment. An important component of the eye is the nasolacrimal drainage system, which serves as a conduit for the fluid of the eye, called tears. Tears flow from the external eye to the nasal cavity by the lacrimal apparatus, which is composed of the structures involved in tear production (figure 3.5). The lacrimal gland, above the eye, secretes tears to keep the eye moist. There are two small openings, one on the inside edge of the upper eyelid and one on the inside edge of the lower eyelid, near the nose. Each of these openings is called a lacrimal punctum. Together, these lacrimal puncta collect tears from the eye that are then conveyed through lacrimal ducts to a reservoir for tears called the lacrimal sac, also known as the dacryocyst or tear sac.
From the sac, tear fluid flows via a nasolacrimal duct to the inner nose. Each nasolacrimal duct is located underneath the skin and passes through the bones of the face into the nose. Chemicals in tears, such as defensins, lactoferrin, and lysozyme, help to prevent colonization by pathogens. In addition, mucins facilitate the removal of microbes from the surface of the eye.
The surfaces of the eyeball and inner eyelid are mucous membranes called conjunctiva. The normal conjunctival microbiota has not been well characterized, but does exist. One small study (part of the Ocular Microbiome project) found twelve genera that were consistently present in the conjunctiva.[4] These microbes are thought to help defend the membranes against pathogens. However, it is still unclear which microbes may be transient and which may form a stable microbiota.[5]
Use of contact lenses can cause changes in the normal microbiota of the conjunctiva by introducing another surface into the natural anatomy of the eye. Research is currently underway to better understand how contact lenses may impact the normal microbiota and contribute to eye disease.
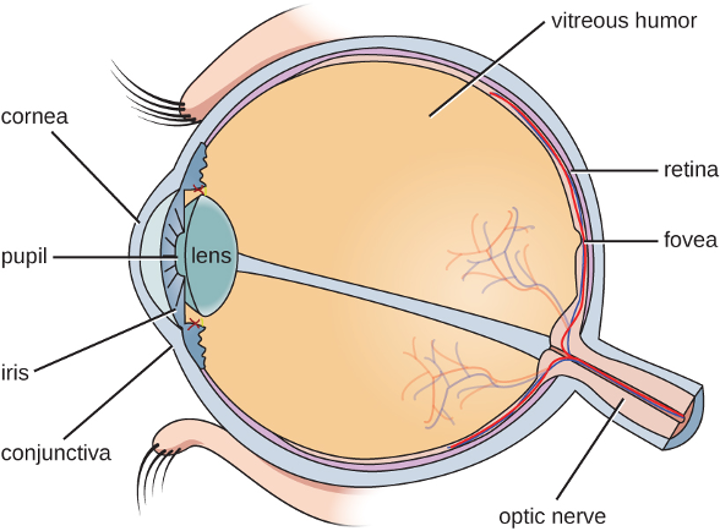
The watery material inside of the eyeball is called vitreous humor. Unlike the conjunctiva, it is protected from contact with the environment and is almost always sterile, with no normal microbiota (figure 3.6).
Infections of the Eye
The conjunctiva is a frequent site of infection of the eye; like other mucous membranes, it is also a common portal of entry for pathogens. Inflammation of the conjunctiva is called conjunctivitis, although it is commonly known as pinkeye because of the pink appearance in the eye. Infections of deeper structures, beneath the cornea, are less common (figure 3.7). Conjunctivitis occurs in multiple forms. It may be acute or chronic. Acute purulent conjunctivitis is associated with pus formation, while acute hemorrhagic conjunctivitis is associated with bleeding in the conjunctiva. The term blepharitis refers to an inflammation of the eyelids, while keratitis refers to an inflammation of the cornea (figure 3.7); keratoconjunctivitis is an inflammation of both the cornea and the conjunctiva, and dacryocystitis is an inflammation of the lacrimal sac that can often occur when a nasolacrimal duct is blocked.
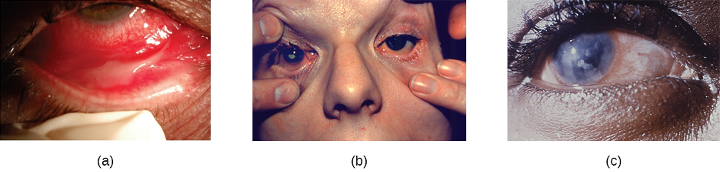
Infections leading to conjunctivitis, blepharitis, keratoconjunctivitis, or dacryocystitis may be caused by bacteria or viruses. Allergens, pollutants, or chemicals can also irritate the eye and cause inflammation of various structures. Viral infection is a more likely cause of conjunctivitis in cases with symptoms such as fever and watery discharge that occurs with upper respiratory infection and itchy eyes. Table 3.2 summarizes some common forms of conjunctivitis and blepharitis.
| Condition | Description | Causative Agent(s) |
|---|---|---|
| Acute purulent conjunctivitis | Conjunctivitis with purulent discharge | Bacterial (Haemophilus, Staphylococcus) |
| Acute hemorrhagic conjunctivitis | Involves subconjunctival hemorrhages | Viral (Picornaviridae) |
| Acute ulcerative blepharitis | Infection involving eyelids; pustules and ulcers may develop | Bacterial (Staphylococcal) or viral (herpes simplex, varicella-zoster, etc.) |
| Follicular conjunctivitis | Inflammation of the conjunctiva with nodules (dome-shaped structures that are red at the base and pale on top) | Viral (adenovirus and others); environmental irritants |
| Dacryocystitis | Inflammation of the lacrimal sac often associated with a plugged nasolacrimal duct | Bacterial (Haemophilus, Staphylococcus, Streptococcus) |
| Keratitis | Inflammation of cornea | Bacterial, viral, or protozoal; environmental irritants |
| Keratoconjunctivitis | Inflammation of cornea and conjunctiva | Bacterial, viral (adenoviruses), or other causes (including dryness of the eye) |
| Nonulcerative blepharitis | Inflammation, irritation, redness of the eyelids without ulceration | Environmental irritants; allergens |
| Papillary conjunctivitis | Inflammation of the conjunctiva; nodules and papillae with red tops develop | Environmental irritants; allergens |
Table 3.2: Types of conjunctivitis and blepharitis
3.2 Bacterial Infections of the Skin and Eyes
Despite the skin’s protective functions, infections are common. Gram-positive Staphylococcus spp. and Streptococcus spp. are responsible for many of the most common skin infections. However, many skin conditions are not strictly associated with a single pathogen. Opportunistic pathogens of many types may infect skin wounds, and individual cases with identical symptoms may result from different pathogens or combinations of pathogens.
In this section, we will examine some of the most important bacterial infections of the skin and eyes and discuss how biofilms can contribute to and exacerbate such infections. Key features of bacterial skin and eye infections are also summarized in tables 3.3 and 3.4.
Staphylococcal Infections of the Skin
Staphylococcus species are commonly found on the skin, with S. epidermidis and S. hominis being prevalent in the normal microbiota. S. aureus is also commonly found in the nasal passages and on healthy skin, but pathogenic strains are often the cause of a broad range of infections of the skin and other body systems.
S. aureus is quite contagious. It is spread easily through skin-to-skin contact, and because many people are chronic nasal carriers (asymptomatic individuals who carry S. aureus in their nares), the bacteria can easily be transferred from the nose to the hands and then to fomites or other individuals. Because it is so contagious, S. aureus is prevalent in most community settings. This prevalence is particularly problematic in hospitals, where antibiotic-resistant strains of the bacteria may be present, and where immunocompromised patients may be more susceptible to infection. Resistant strains include methicillin-resistant S. aureus (MRSA), which can be acquired through healthcare settings (hospital-acquired MRSA, or HA-MRSA) or in the community (community-acquired MRSA, or CA-MRSA). Hospital patients often arrive at healthcare facilities already colonized with antibiotic-resistant strains of S. aureus that can be transferred to healthcare providers and other patients. Some hospitals have attempted to detect these individuals in order to institute prophylactic measures, but they have had mixed success.
When a staphylococcal infection develops, choice of medication is important. As discussed above, many staphylococci (such as MRSA) are resistant to some or many antibiotics. Thus, antibiotic sensitivity is measured to identify the most suitable antibiotic. However, even before receiving the results of sensitivity analysis, suspected S. aureus infections are often initially treated with drugs known to be effective against MRSA, such as trimethoprim-sulfamethoxazole (TMP/SMZ), clindamycin, a tetracycline (doxycycline or minocycline), or linezolid.
The pathogenicity of staphylococcal infections is often enhanced by characteristic chemicals secreted by some strains. Staphylococcal virulence factors include hemolysins called staphylolysin, which are cytotoxic for many types of cells, including skin cells and white blood cells. Virulent strains of S. aureus are also coagulase-positive, meaning they produce coagulase, a plasma-clotting protein that is involved in abscess formation. They may also produce leukocidins, which kill white blood cells and can contribute to the production of pus and Protein A, which inhibits phagocytosis by binding to the constant region of antibodies. Some virulent strains of S. aureus also produce other toxins, such as toxic shock syndrome toxin-1 (see section 2.14).
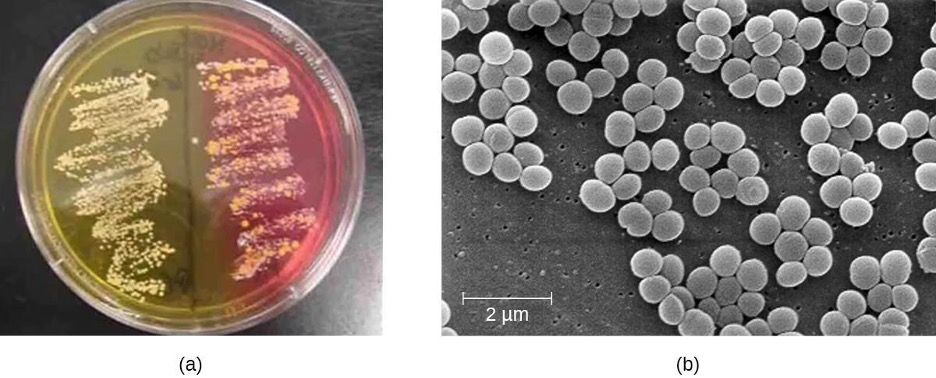
To confirm the causative agent of a suspected staphylococcal skin infection, samples from the wound are cultured. Under the microscope, gram-positive Staphylococcus species have cellular arrangements that form grapelike clusters; when grown on blood agar, colonies have a unique pigmentation ranging from opaque white to cream. A catalase test is used to distinguish Staphylococcus from Streptococcus, which is also a genus of gram-positive cocci and a common cause of skin infections. Staphylococcus species are catalase-positive while Streptococcus species are catalase-negative.
Other tests are performed on samples from the wound in order to distinguish coagulase-positive species of Staphylococcus (CoPS) such as S. aureus from common coagulase-negative species (CoNS) such as S. epidermidis. Although CoNS are less likely than CoPS to cause human disease, they can cause infections when they enter the body, as can sometimes occur via catheters, indwelling medical devices, and wounds. Passive agglutination testing can be used to distinguish CoPS from CoNS. If the sample is coagulase-positive, the sample is generally presumed to contain S. aureus. Additional genetic testing would be necessary to identify the particular strain of S. aureus.
Another way to distinguish CoPS from CoNS is by culturing the sample on mannitol salt agar (MSA). Staphylococcus species readily grow on this medium because they are tolerant of the high concentration of sodium chloride (7.5% NaCl). However, CoPS such as S. aureus ferment mannitol (which will be evident on a MSA plate). Conversely, CoNS such as S. epidermidis do not ferment mannitol but can be distinguished by the fermentation of other sugars such as lactose, malonate, and raffinose (figure 3.8).
Superficial Staphylococcal Infections
S. aureus is often associated with pyoderma, skin infections that are purulent. Pus formation occurs because many strains of S. aureus produce leukocidins, which kill white blood cells. These purulent skin infections may initially manifest as folliculitis, but can lead to furuncles or deeper abscesses called carbuncles.
Folliculitis generally presents as bumps and pimples that may be itchy, red, and/or pus-filled. In some cases, folliculitis is self-limiting, but if it continues for more than a few days, worsens, or returns repeatedly, it may require medical treatment. Sweat, skin injuries, ingrown hairs, tight clothing, irritation from shaving, and skin conditions can all contribute to folliculitis. Avoidance of tight clothing and skin irritation can help to prevent infection, but topical antibiotics (and sometimes other treatments) may also help. Folliculitis can be identified by skin inspection; treatment is generally started without first culturing and identifying the causative agent.
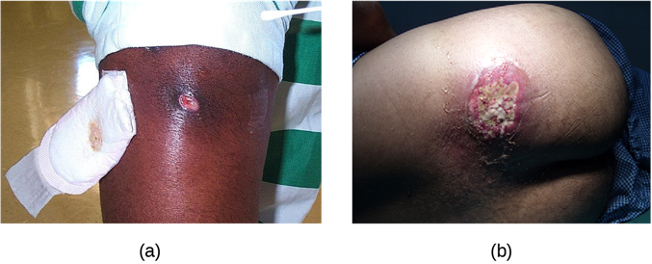
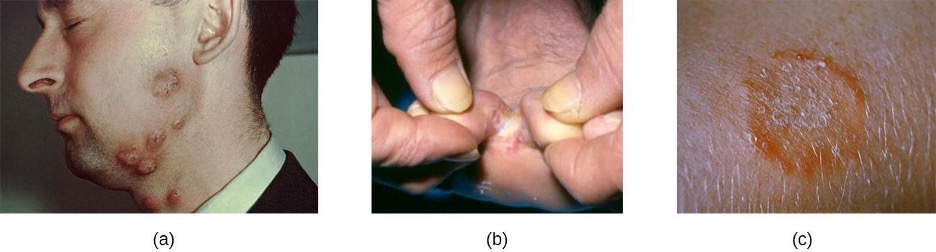
In contrast, furuncles (boils) are deeper infections (figure 3.9). They are most common in those individuals (especially young adults and teenagers) who play contact sports, share athletic equipment, have poor nutrition, live in close quarters, or have weakened immune systems. Good hygiene and skin care can often help to prevent furuncles from becoming more infective, and they generally resolve on their own. However, if furuncles spread, increase in number or size, or lead to systemic symptoms such as fever and chills, then medical care is needed. They may sometimes need to be drained (at which time the pathogens can be cultured) and treated with antibiotics.
When multiple boils develop into a deeper lesion, it is called a carbuncle (figure 3.9). Because carbuncles are deeper, they are more commonly associated with systemic symptoms and a general feeling of illness. Larger, recurrent, or worsening carbuncles require medical treatment, as do those associated with signs of illness such as fever. Carbuncles generally need to be drained and treated with antibiotics. While carbuncles are relatively easy to identify visually, culturing and laboratory analysis of the wound may be recommended for some infections because antibiotic resistance is relatively common.
Proper hygiene is important to prevent these types of skin infections or to prevent the progression of existing infections.
Staphylococcal scalded skin syndrome (SSSS) is another superficial infection caused by S. aureus that is most commonly seen in young children, especially infants. Bacterial exotoxins first produce erythema (redness of the skin) and then severe peeling of the skin, as might occur after scalding (figure 3.10). SSSS is diagnosed by examining characteristics of the skin (which may rub off easily), using blood tests to check for elevated white blood cell counts, culturing, and other methods. Intravenous antibiotics and fluid therapy are used as treatment.
Impetigo
The skin infection impetigo causes the formation of vesicles, pustules, and possibly bullae, often around the nose and mouth. Bullae are large, fluid-filled blisters that measure at least 5 mm in diameter. Impetigo can be diagnosed as either nonbullous or bullous. In nonbullous impetigo, vesicles and pustules rupture and become encrusted sores. Typically the crust is yellowish, often with exudate draining from the base of the lesion. In bullous impetigo, the bullae fill and rupture, resulting in larger, draining, encrusted lesions (figure 3.11).
Especially common in children, impetigo is particularly concerning because it is highly contagious. Impetigo can be caused by S. aureus alone, by Streptococcus pyogenes alone, or by coinfection of S. aureus and S. pyogenes. Impetigo is often diagnosed through observation of its characteristic appearance, although culture and susceptibility testing may also be used.
Topical or oral antibiotic treatment is typically effective in treating most cases of impetigo. However, cases caused by S. pyogenes can lead to serious sequelae (pathological conditions resulting from infection, disease, injury, therapy, or other trauma) such as acute glomerulonephritis (AGN), which is severe inflammation in the kidneys.
Nosocomial S. epidermidis Infections

Though not as virulent as S. aureus, the staphylococcus S. epidermidis can cause serious opportunistic infections. Such infections usually occur only in hospital settings. S. epidermidis is usually a harmless resident of the normal skin microbiota. However, healthcare workers can inadvertently transfer S. epidermidis to medical devices that are inserted into the body, such as catheters, prostheses, and indwelling medical devices. Once it has bypassed the skin barrier, S. epidermidis can cause infections inside the body that can be difficult to treat. Like S. aureus, S. epidermidis is resistant to many antibiotics, and localized infections can become systemic if not treated quickly. To reduce the risk of nosocomial (hospital-acquired) S. epidermidis, health-care workers must follow strict procedures for handling and sterilizing medical devices before and during surgical procedures.
Streptococcal Infections of the Skin
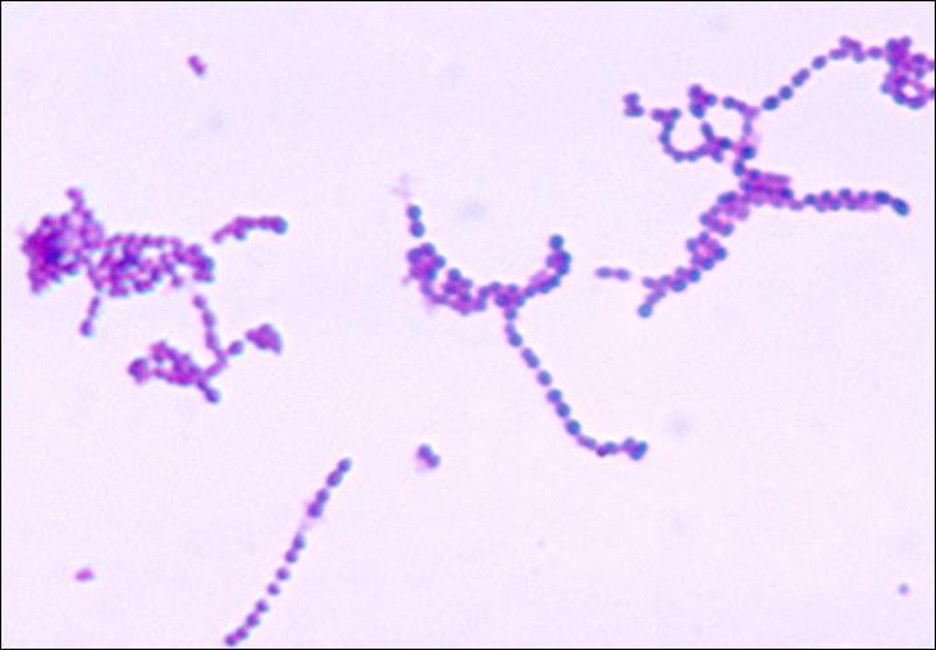
Streptococcus are gram-positive cocci with a microscopic morphology that resembles chains of bacteria. Colonies are typically small (1–2 mm in diameter), translucent, entire edge, with a slightly raised elevation that can be either nonhemolytic, alpha-hemolytic, or beta-hemolytic when grown on blood agar (figure 3.12). Additionally, they are facultative anaerobes that are catalase-negative.
The genus Streptococcus includes important pathogens that are categorized in serological Lancefield groups based on the distinguishing characteristics of their surface carbohydrates. The most clinically important streptococcal species in humans is S. pyogenes, also known as group A streptococcus (GAS). S. pyogenes produces a variety of extracellular enzymes, including streptolysins O and S, hyaluronidase, and streptokinase. These enzymes can aid in transmission and contribute to the inflammatory response.[6] S. pyogenes also produces a capsule and M protein, a streptococcal cell wall protein. These virulence factors help the bacteria to avoid phagocytosis while provoking a substantial immune response that contributes to symptoms associated with streptococcal infections.
S. pyogenes causes a wide variety of diseases not only in the skin, but in other organ systems as well. Examples of diseases elsewhere in the body include pharyngitis and scarlet fever.
Cellulitis, Erysipelas, and Erythema Nodosum
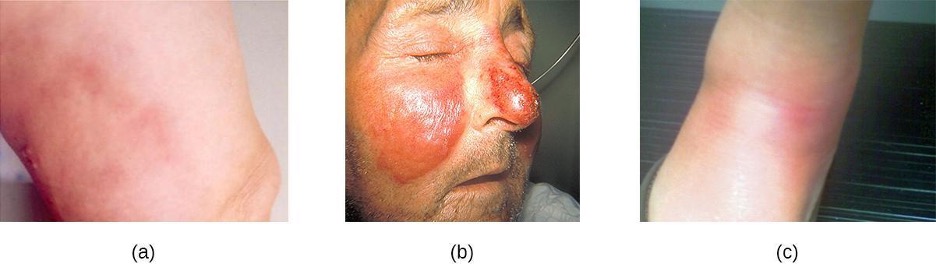
Common streptococcal conditions of the skin include cellulitis, erysipelas, and erythema nodosum. An infection that develops in the dermis or hypodermis can cause cellulitis, which presents as a reddened area of the skin that is warm to the touch and painful. The causative agent is often S. pyogenes, which may breach the epidermis through a cut or abrasion, although cellulitis may also be caused by staphylococci. S. pyogenes can also cause erysipelas, a condition that presents as a large, intensely inflamed patch of skin involving the dermis (often on the legs or face). These infections can be suppurative, which results in a bullous form of erysipelas. Streptococcal and other pathogens may also cause a condition called erythema nodosum, characterized by inflammation in the subcutaneous fat cells of the hypodermis. It sometimes results from a streptococcal infection, though other pathogens can also cause the condition. It is not suppurative, but leads to red nodules on the skin, most frequently on the shins (figure 3.13).
In general, streptococcal infections are best treated through identification of the specific pathogen followed by treatment based upon that particular pathogen’s susceptibility to different antibiotics. Many immunological tests, including agglutination reactions and ELISAs, can be used to detect streptococci. Penicillin is commonly prescribed for treatment of cellulitis and erysipelas because resistance is not widespread in streptococci at this time. In most patients, erythema nodosum is self-limiting and is not treated with antimicrobial drugs. Recommended treatments may include nonsteroidal anti-inflammatory drugs (NSAIDs), cool wet compresses, elevation, and bed rest.
Necrotizing Fasciitis
Streptococcal infections that start in the skin can sometimes spread elsewhere, resulting in a rare but potentially life-threatening condition called necrotizing fasciitis, sometimes referred to as flesh-eating bacterial syndrome. S. pyogenes is one of several species that can cause this rare but potentially-fatal condition; others include Klebsiella, Clostridium, Escherichia coli, S. aureus, and Aeromonas hydrophila.
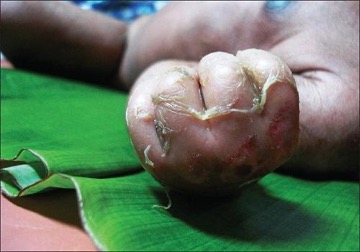
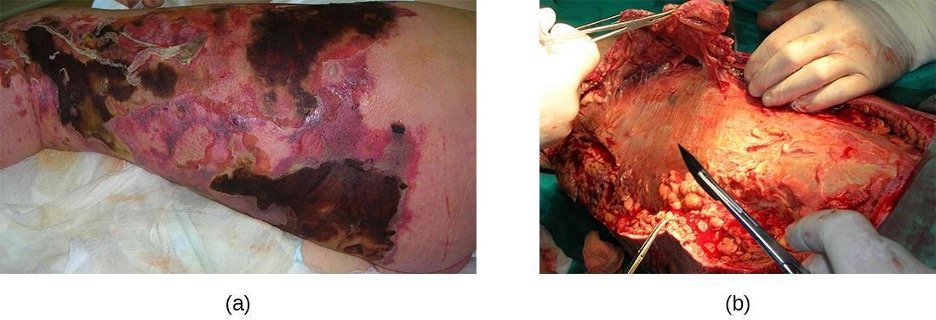
Necrotizing fasciitis occurs when the fascia, a thin layer of connective tissue between the skin and muscle, becomes infected. Severe invasive necrotizing fasciitis due to Streptococcus pyogenes occurs when virulence factors that are responsible for adhesion and invasion overcome host defenses. S. pyogenes invasions allow bacterial cells to adhere to tissues and establish infection. Bacterial proteases unique to S. pyogenes aggressively infiltrate and destroy host tissues, inactivate complement, and prevent neutrophil migration to the site of infection. The infection and resulting tissue death can spread very rapidly, as large areas of skin become detached and die. Treatment generally requires debridement (surgical removal of dead or infected tissue) or amputation of infected limbs to stop the spread of the infection; surgical treatment is supplemented with intravenous antibiotics and other therapies (figure 3.14).
Necrotizing fasciitis does not always originate from a skin infection; in some cases there is no known portal of entry. Some studies have suggested that experiencing a blunt force trauma can increase the risk of developing streptococcal necrotizing fasciitis.[7]
Pseudomonas Infections of the Skin
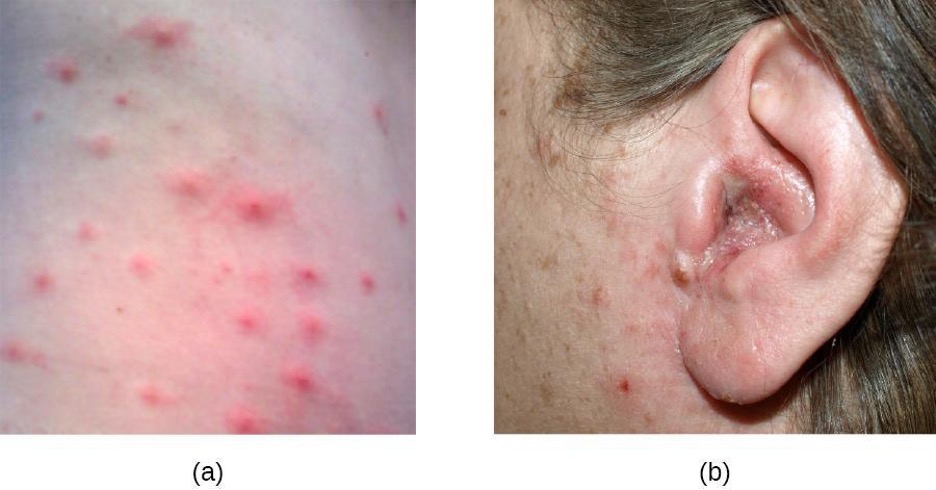
Another important skin pathogen is Pseudomonas aeruginosa, a gram-negative, oxidase-positive, aerobic bacillus that is commonly found in water and soil as well as on human skin. P. aeruginosa is a common cause of opportunistic infections of wounds and burns. It can also cause hot tub rash, a condition characterized by folliculitis that frequently afflicts users of pools and hot tubs. P. aeruginosa is also the cause of otitis externa (swimmer’s ear), an infection of the ear canal that causes itching, redness, and discomfort, and can progress to fever, pain, and swelling (figure 3.15).
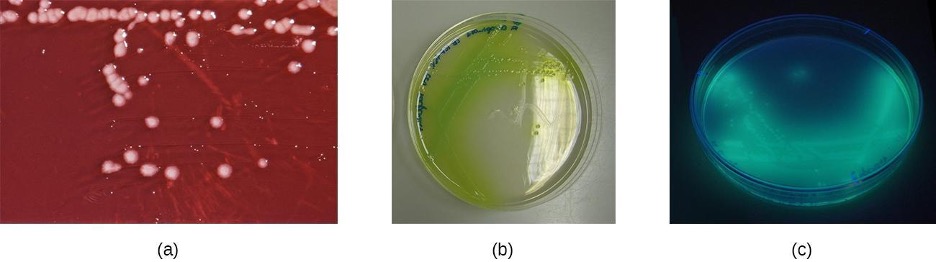
Wounds infected with P. aeruginosa have a distinctive odor resembling grape soda or fresh corn tortillas. This odor is caused by the 2-aminoacetophenone that is used by P. aeruginosa in quorum sensing and contributes to its pathogenicity. Wounds infected with certain strains of P. aeruginosa also produce a blue-green pus due to the pigments pyocyanin and pyoverdin, which also contribute to its virulence. Pyocyanin and pyoverdin are siderophores that help P. aeruginosa survive in low-iron environments by enhancing iron uptake. P. aeruginosa also produces several other virulence factors, including phospholipase C (a hemolysin capable of breaking down red blood cells), exoenzyme S (involved in adherence to epithelial cells), and exotoxin A (capable of causing tissue necrosis). Other virulence factors include a slime that allows the bacterium to avoid being phagocytized, fimbriae for adherence, and proteases that cause tissue damage. P. aeruginosa can be detected through the use of cetrimide agar, which is selective for Pseudomonas species (figure 3.16).
Pseudomonas spp. tend to be resistant to most antibiotics. They often produce β-lactamases, may have mutations affecting porins (small cell wall channels) that limit antibiotic uptake, and may pump some antibiotics out of the cell, contributing to this resistance. Polymyxin B and gentamicin are effective, as are some fluoroquinolones. Otitis externa is typically treated with ear drops containing acetic acid, antibacterials, and/or steroids to reduce inflammation; ear drops may also include antifungals because fungi can sometimes cause or contribute to otitis externa. Wound infections caused by Pseudomonas spp. may be treated with topical antibiofilm agents that disrupt the formation of biofilms.
Acne
One of the most ubiquitous skin conditions is acne. Acne afflicts nearly 80% of teenagers and young adults, but it can be found in individuals of all ages. Higher incidence among adolescents is due to hormonal changes that can result in overproduction of sebum.
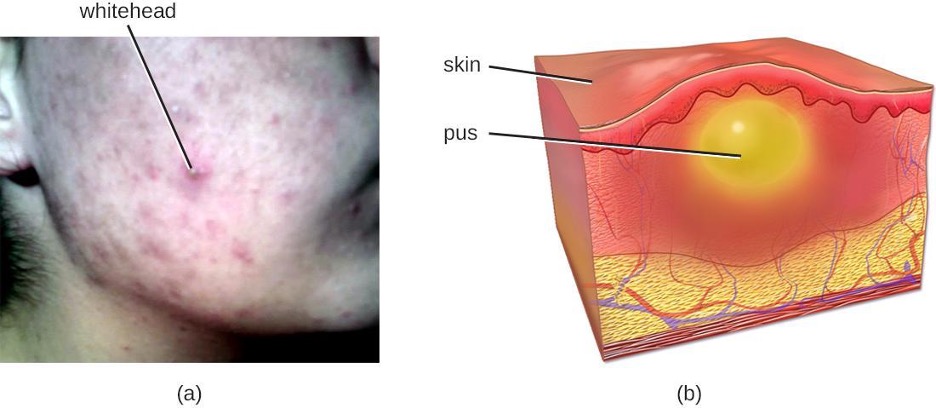
Acne occurs when hair follicles become clogged by shed skin cells and sebum, causing non-inflammatory lesions called comedones. Comedones (singular “comedo”) can take the form of whitehead and blackhead pimples. Whiteheads are covered by skin, whereas blackhead pimples are not; the black color occurs when lipids in the clogged follicle become exposed to the air and oxidize (figure 3.17).
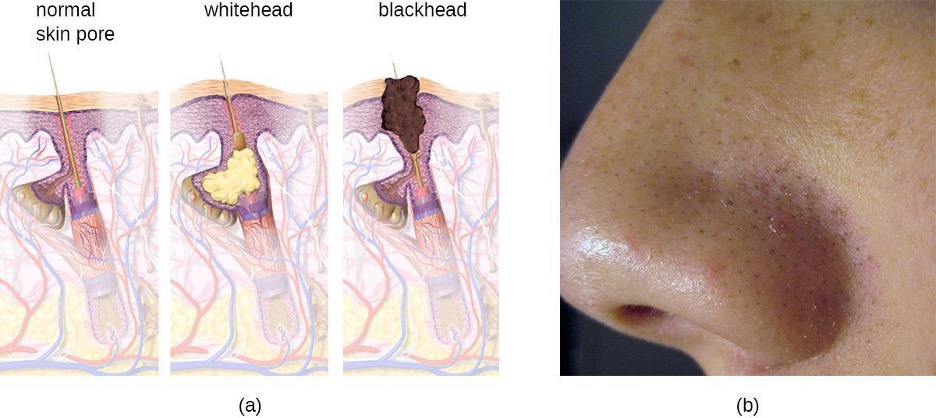
Often comedones lead to infection by Propionibacterium acnes, a gram-positive, non-spore-forming, aerotolerant anaerobic bacillus found on skin that consumes components of sebum. P. acnes secretes enzymes that damage the hair follicle, causing inflammatory lesions that may include papules, pustules, nodules, or pseudocysts, depending on their size and severity.
Treatment of acne depends on the severity of the case. There are multiple ways to grade acne severity, but three levels are usually considered based on the number of comedones, the number of inflammatory lesions, and the types of lesions. Mild acne is treated with topical agents that may include salicylic acid (which helps to remove old skin cells) or retinoids (which have multiple mechanisms, including the reduction of inflammation). Moderate acne may be treated with antibiotics (erythromycin, clindamycin), acne creams (e.g., benzoyl peroxide), and hormones. Severe acne may require treatment using strong medications such as isotretinoin (a retinoid that reduces oil buildup, among other effects, but that also has serious side effects such as photosensitivity). Other treatments, such as phototherapy and laser therapy to kill bacteria and possibly reduce oil production, are also sometimes used.
Anthrax
The zoonotic disease anthrax is caused by Bacillus anthracis, a gram-positive, endospore-forming, facultative anaerobe. Anthrax mainly affects animals such as sheep, goats, cattle, and deer, but can be found in humans as well. Sometimes called wool sorter’s disease, it is often transmitted to humans through contact with infected animals or animal products, such as wool or hides. However, exposure to B. anthracis can occur by other means, as the endospores are widespread in soils and can survive for long periods of time, sometimes for hundreds of years.
The vast majority of anthrax cases (95–99%) occur when anthrax endospores enter the body through abrasions of the skin.[8] This form of the disease is called cutaneous anthrax. It is characterized by the formation of a nodule on the skin; the cells within the nodule die, forming a black eschar, a mass of dead skin tissue (figure 3.18). The localized infection can eventually lead to bacteremia and septicemia. If untreated, cutaneous anthrax can cause death in 20% of patients.[9] Once in the skin tissues, B. anthracis endospores germinate and produce a capsule, which prevents the bacteria from being phagocytized, and two binary exotoxins that cause edema and tissue damage. The first of the two exotoxins consists of a combination of protective antigen (PA) and an enzymatic lethal factor (LF), forming lethal toxin (LeTX). The second consists of protective antigen (PA) and an edema factor (EF), forming edema toxin (EdTX).
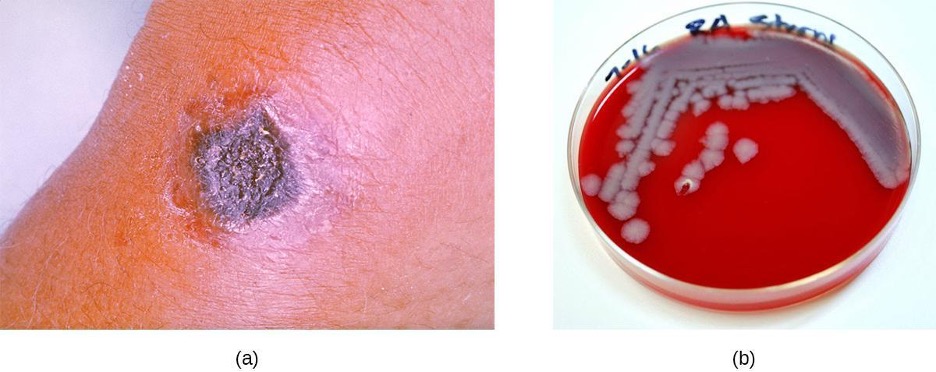
Less commonly, anthrax infections can be initiated through other portals of entry such as the digestive tract (gastrointestinal anthrax) or respiratory tract (pulmonary anthrax or inhalation anthrax). Typically, cases of noncutaneous anthrax are more difficult to treat than the cutaneous form. The mortality rate for gastrointestinal anthrax can be up to 40%, even with treatment. Inhalation anthrax, which occurs when anthrax spores are inhaled, initially causes influenza-like symptoms, but mortality rates are approximately 45% in treated individuals and 85% in those not treated. A relatively new form of the disease, injection anthrax, has been reported in Europe in intravenous drug users; it occurs when drugs are contaminated with B. anthracis. Patients with injection anthrax show signs and symptoms of severe soft tissue infection that differ clinically from cutaneous anthrax. This often delays diagnosis and treatment, and leads to a high mortality rate.[10]
B. anthracis colonies on blood agar have a rough texture and serrated edges that eventually form an undulating band (figure 3.18). Broad spectrum antibiotics such as penicillin, erythromycin, and tetracycline are often effective treatments.
Unfortunately, B. anthracis has been used as a biological weapon and remains on the United Nations’ list of potential agents of bioterrorism.[11] Over a period of several months in 2001, a number of letters were mailed to members of the news media and the United States Congress. As a result, 11 individuals developed cutaneous anthrax and another 11 developed inhalation anthrax. Those infected included recipients of the letters, postal workers, and two other individuals. Five of those infected with pulmonary anthrax died. The anthrax spores had been carefully prepared to aerosolize, showing that the perpetrator had a high level of expertise in microbiology.[12]
A vaccine is available to protect individuals from anthrax. However, unlike most routine vaccines, the current anthrax vaccine is unique in both its formulation and the protocols dictating who receives it.[13] The vaccine is administered through five intramuscular injections over a period of 18 months, followed by annual boosters. The US Food and Drug Administration (FDA) has only approved administration of the vaccine prior to exposure for at-risk adults, such as individuals who work with anthrax in a laboratory, some individuals who handle animals or animal products (e.g., some veterinarians), and some members of the United States military. The vaccine protects against cutaneous and inhalation anthrax using cell-free filtrates of microaerophilic cultures of an avirulent, nonencapsulated strain of B. anthracis.[14] The FDA has not approved the vaccine for routine use after exposure to anthrax, but if there were ever an anthrax emergency in the United States, patients could be given anthrax vaccine after exposure to help prevent disease.
| Disease | Pathogen | Signs and Symptoms | Transmission | Antimicrobial Drugs |
|---|---|---|---|---|
| Acne | Propionibacterium acnes | Comedones (whiteheads, blackheads); papules, pustules, nodules, or pseudocysts | Not transmissible; clogged pores become infected by normal skin microbiota (P. acnes) | Erythromycin, clindamycin |
| Anthrax (cutaneous) | Bacillus anthracis | Eschar at site of infection; may lead to septicemia and can be fatal | Entry of B. anthracis endospores through cut or abrasion | Penicillin, erythromycin, or tetracycline |
| Cellulitis | Streptococcus pyogenes | Localized inflammation of dermis and hypodermis; skin red, warm, and painful to the touch | Entry of S. pyogenes through cut or abrasion | Oral or intravenous antibiotics (e.g., penicillin) |
| Erysipelas | S. pyogenes | Inflamed, swollen patch of skin, often on face; may be suppurative | Entry of S. pyogenes through cut or abrasion | Oral or intravenous antibiotics (e.g., penicillin) |
| Erythema nodosum | S. pyogenes | Small red nodules, often on shins | Associated with other streptococcal infection | None or anti-inflammatory drugs for severe cases |
| Impetigo | Staphylococcus aureus, S. pyogenes | Vesicles, pustules, and sometimes bullae around nose and mouth | Highly contagious, especially via contact | Topical or oral antibiotics. |
| Necrotizing fasciitis | S. pyogenes, Klebsiella, Clostridium, others | Infection of fascia and rapidly spreading tissue death; can lead to septic shock and death | Entry of bacteria through cut or abrasion | Intravenous broad-spectrum antibiotics |
| Otitis externa | Pseudomonas aeruginosa | Itching, redness, discomfort of ear canal, progressing to fever, pain, swelling | P. aeruginosa enters ear canal via pool or other water | Acidic ear drops with antibiotics, antifungals, steroids |
| Staphylococcal scalded skin syndrome (SSSS) | S. aureus | Erythema and severe peeling of skin | Infection of skin and mucous membranes, especially in children | Intravenous antibiotics, fluid therapy |
| Wound infections | P. aeruginosa, others | Formation of biofilm in or on wound | Exposure of wound to microbes in environment; poor wound hygiene | Polymyxin B, gentamicin, fluoroquinolones, topical anti-biofilm agents. |
Table 3.3: Bacterial infections of the skin
Bacterial Conjunctivitis
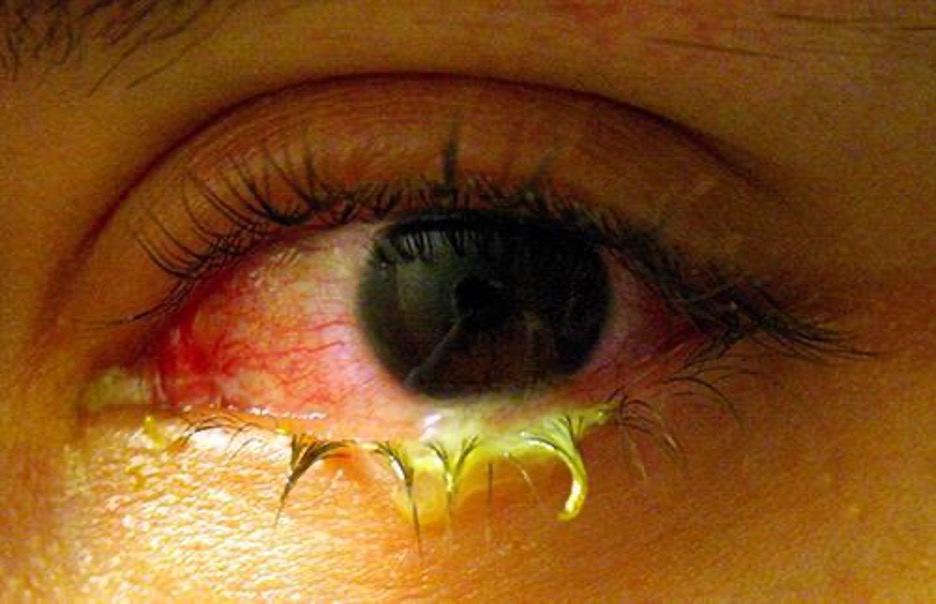
Like the skin, the surface of the eye comes in contact with the outside world and is somewhat prone to infection by bacteria in the environment. Bacterial conjunctivitis (pinkeye) is a condition characterized by inflammation of the conjunctiva, often accompanied by a discharge of sticky fluid (described as acute purulent conjunctivitis) (figure 3.19). Conjunctivitis can affect one eye or both, and it usually does not affect vision permanently. Bacterial conjunctivitis is most commonly caused by Haemophilus influenzae, but can also be caused by other species such as Moraxella catarrhalis, S. pneumoniae, and S. aureus. The causative agent may be identified using bacterial cultures, Gram stain, and diagnostic biochemical, antigenic, or nucleic acid profile tests of the isolated pathogen. Bacterial conjunctivitis is very contagious, being transmitted via secretions from infected individuals, but it is also self-limiting. Bacterial conjunctivitis usually resolves in a few days, but topical antibiotics are sometimes prescribed. Because this condition is so contagious, medical attention is recommended whenever it is suspected. Individuals who use contact lenses should discontinue their use when conjunctivitis is suspected. Certain symptoms, such as blurred vision, eye pain, and light sensitivity, can be associated with serious conditions and require medical attention.
Neonatal Conjunctivitis
Newborns whose mothers have certain sexually transmitted infections are at risk of contracting ophthalmia neonatorum or inclusion conjunctivitis, which are two forms of neonatal conjunctivitis contracted through exposure to pathogens during passage through the birth canal. Gonococcal ophthalmia neonatorum is caused by Neisseria gonorrhoeae, the bacterium that causes the STD gonorrhea (figure 3.20). Inclusion (chlamydial) conjunctivitis is caused by Chlamydia trachomatis, the anaerobic, obligate, intracellular parasite that causes the STD chlamydia.
To prevent gonococcal ophthalmia neonatorum, silver nitrate ointments were once routinely applied to all infants’ eyes shortly after birth; however, it is now more common to apply antibacterial creams or drops, such as erythromycin. Most hospitals are required by law to provide this preventative treatment to all infants, because conjunctivitis caused by N. gonorrhoeae, C. trachomatis, or other bacteria acquired during a vaginal delivery can have serious complications. If untreated, the infection can spread to the cornea, resulting in ulceration or perforation that can cause vision loss or even permanent blindness. As such, neonatal conjunctivitis is treated aggressively with oral or intravenous antibiotics to stop the spread of the infection. Causative agents of inclusion conjunctivitis may be identified using bacterial cultures, Gram stain, and diagnostic biochemical, antigenic, or nucleic acid profile tests.
Trachoma
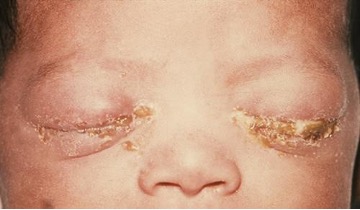
Trachoma, or granular conjunctivitis, is a common cause of preventable blindness that is rare in the United States but widespread in developing countries, especially in Africa and Asia. The condition is caused by the same species that causes neonatal inclusion conjunctivitis in infants, Chlamydia trachomatis. C. trachomatis can be transmitted easily through fomites such as contaminated towels, bed linens, and clothing and also by direct contact with infected individuals. C. trachomatis can also be spread by flies that transfer infected mucous containing C. trachomatis from one human to another.
Infection by C. trachomatis causes chronic conjunctivitis, which leads to the formation of necrotic follicles and scarring in the upper eyelid. The scars turn the eyelashes inward (a condition known as trichiasis) and mechanical abrasion of the cornea leads to blindness (figure 3.21). Antibiotics such as azithromycin are effective in treating trachoma, and outcomes are good when the disease is treated promptly. In areas where this disease is common, large public health efforts are focused on reducing transmission by teaching people how to avoid the risks of the infection.
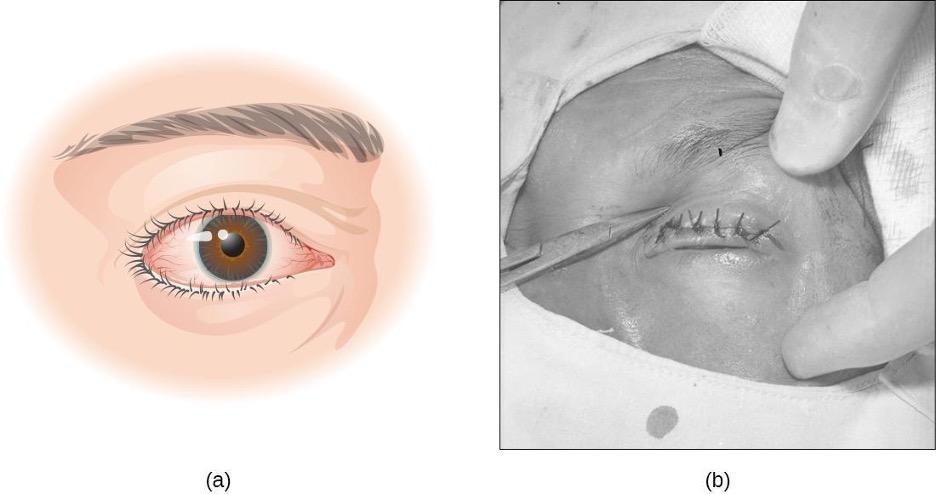
Bacterial Keratitis
Keratitis can have many causes, but bacterial keratitis is most frequently caused by Staphylococcus epidermidis and/or Pseudomonas aeruginosa. Contact lens users are particularly at risk for such an infection because S. epidermidis and P. aeruginosa both adhere well to the surface of the lenses. Risk of infection can be greatly reduced by proper care of contact lenses and avoiding wearing lenses overnight. Because the infection can quickly lead to blindness, prompt and aggressive treatment with antibiotics is important. The causative agent may be identified using bacterial cultures, Gram stain, and diagnostic biochemical, antigenic, or nucleic acid profile tests of the isolated pathogen.
Biofilms and Infections of the Skin and Eyes
When treating bacterial infections of the skin and eyes, it is important to consider that few such infections can be attributed to a single pathogen. While biofilms may develop in other parts of the body, they are especially relevant to skin infections (such as those caused by S. aureus or P. aeruginosa) because of their prevalence in chronic skin wounds. Biofilms develop when bacteria (and sometimes fungi) attach to a surface and produce extracellular polymeric substances (EPS) in which cells of multiple organisms may be embedded. When a biofilm develops on a wound, it may interfere with the natural healing process as well as diagnosis and treatment.
Because biofilms vary in composition and are difficult to replicate in the lab, they are still not thoroughly understood. The extracellular matrix of a biofilm consists of polymers such as polysaccharides, extracellular DNA, proteins, and lipids, but the exact makeup varies. The organisms living within the extracellular matrix may include familiar pathogens as well as other bacteria that do not grow well in cultures (such as numerous obligate anaerobes). This presents challenges when culturing samples from infections that involve a biofilm. Because only some species grow in vitro, the culture may contain only a subset of the bacterial species involved in the infection.
Biofilms confer many advantages to the resident bacteria. For example, biofilms can facilitate attachment to surfaces on or in the host organism (such as wounds), inhibit phagocytosis, prevent the invasion of neutrophils, and sequester host antibodies. Additionally, biofilms can provide a level of antibiotic resistance not found in the isolated cells and colonies that are typical of laboratory cultures. The extracellular matrix provides a physical barrier to antibiotics, shielding the target cells from exposure. Moreover, cells within a biofilm may differentiate to create subpopulations of dormant cells called persister cells. Nutrient limitations deep within a biofilm add another level of resistance, as stress responses can slow metabolism and increase drug resistance.
| Disease | Pathogen | Signs and Symptoms | Transmission | Antimicrobial Drugs |
|---|---|---|---|---|
| Acute bacterial conjunctivitis | Haemophilus influenza | Inflammation of conjunctiva with purulent discharge | Exposure to secretions from infected individuals | Broad-spectrum topical antibiotics |
| Bacterial keratitis | Staphylococcus epidermidis, Pseudomonas aeruginosa | Redness and irritation of eye, blurred vision, sensitivity to light; progressive corneal scarring, which can lead to blindness | Exposure to pathogens on contaminated contact lenses | Antibiotic eye drops (e.g., with fluoroquinolones) |
| Neonatal conjunctivitis | Chlamydia trachomatis, Neisseria gonorrhoeae | Inflammation of conjunctiva, purulent discharge, scarring and perforation of cornea; may lead to blindness | Neonate exposed to pathogens in birth canal of a pregnant person with chlamydia or gonorrhea | Erythromycin |
| Trachoma (granular conjunctivitis) | C. trachomatis | Chronic conjunctivitis, trichiasis, scarring, blindness | Contact with infected individuals or contaminated fomites; transmission by eye-seeking flies | Azithromycin |
Table 3.4: Bacterial infections of the eye
3.3 Viral Infections of the Skin and Eyes
Until recently, it was thought that the normal microbiota of the body consisted primarily of bacteria and some fungi. However, in addition to bacteria, the skin is colonized by viruses, and recent studies suggest that the viral families Papillomaviridae, Polyomaviridae and Circoviridae also contribute to the normal skin microbiota. However, some viruses associated with skin are pathogenic, and these viruses can cause diseases with a wide variety of presentations; common viral infections are summarized in table 3.5.
Numerous types of viral infections cause rashes or lesions on the skin; however, in many cases these skin conditions result from infections that originate in other body systems. In this section, we will limit the discussion to viral skin infections that use the skin as a portal of entry. Later chapters will discuss viral infections such as chickenpox, measles, and rubella—diseases that cause skin rashes but invade the body through portals of entry other than the skin.
Papillomas
Papillomas (warts) are the expression of common skin infections by human papillomavirus (HPV) and are transmitted by direct contact. There are many types of HPV, and they lead to a variety of different presentations, such as common warts, plantar warts, flat warts, and filiform warts. HPV can also cause sexually-transmitted genital warts. Vaccination is available for some strains of HPV.
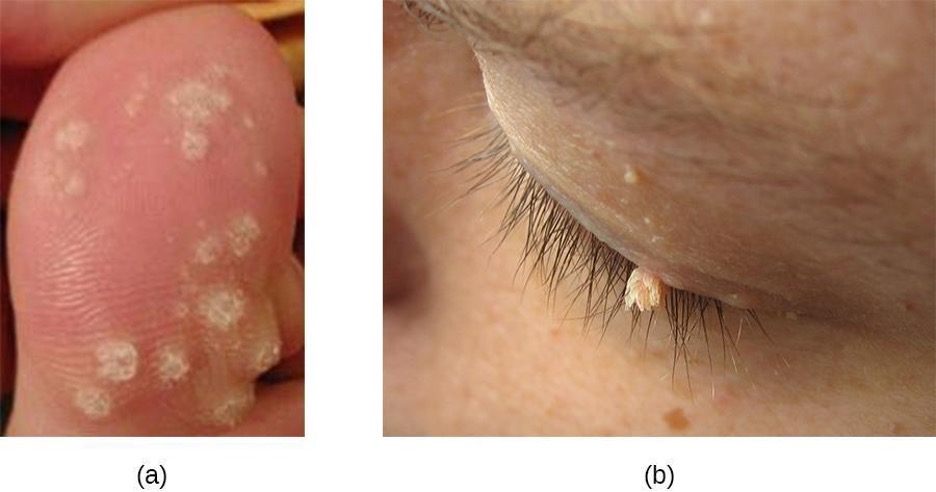
Common warts tend to develop on fingers, the backs of hands, and around nails in areas with broken skin. In contrast, plantar warts (also called foot warts) develop on the sole of the foot and can grow inwards, causing pain and pressure during walking. Flat warts can develop anywhere on the body, are often numerous, and are relatively smooth and small compared with other wart types. Filiform warts are long, threadlike warts that grow quickly.
In some cases, the immune system may be strong enough to prevent warts from forming or to eradicate established warts. However, treatment of established warts is typically required. There are many available treatments for warts, and their effectiveness varies. Common warts can be frozen off with liquid nitrogen. Topical applications of salicylic acid may also be effective. Other options are electrosurgery (burning), curettage (cutting), excision, painting with cantharidin (which causes the wart to die so it can more easily be removed), laser treatments, treatment with bleomycin, chemical peels, and immunotherapy (figure 3.22).
Oral Herpes
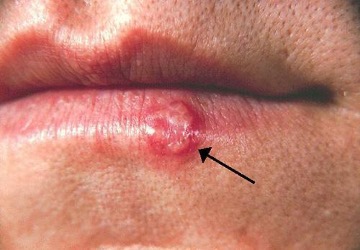
Another common skin virus is herpes simplex virus (HSV). HSV has historically been divided into two types, HSV-1 and HSV-2. HSV-1 is typically transmitted by direct oral contact between individuals, and is usually associated with oral herpes. HSV-2 is usually transmitted sexually and is typically associated with genital herpes. However, both HSV-1 and HSV-2 are capable of infecting any mucous membrane, and the incidence of genital HSV-1 and oral HSV-2 infections has been increasing in recent years. In this section, we will limit our discussion to infections caused by HSV-1; HSV-2 and genital herpes will be discussed in section 7.3.
Infection by HSV-1 commonly manifests as cold sores or fever blisters, usually on or around the lips (figure 3.23). HSV-1 is highly contagious, with some studies suggesting that up to 65% of the US population is infected; however, many infected individuals are asymptomatic.[15] Moreover, the virus can be latent for long periods, residing in the trigeminal nerve ganglia between recurring bouts of symptoms. Recurrence can be triggered by stress or environmental conditions (systemic or affecting the skin). When lesions are present, they may blister, break open, and crust. The virus can be spread through direct contact, even when a patient is asymptomatic.
While the lips, mouth, and face are the most common sites for HSV-1 infections, lesions can spread to other areas of the body. Wrestlers and other athletes involved in contact sports may develop lesions on the neck, shoulders, and trunk. This condition is often called herpes gladiatorum. Herpes lesions that develop on the fingers are often called herpetic whitlow.
HSV-1 infections are commonly diagnosed from their appearance, although laboratory testing can confirm the diagnosis. There is no cure, but antiviral medications such as acyclovir, penciclovir, famciclovir, and valacyclovir are used to reduce symptoms and risk of transmission. Topical medications, such as creams with n-docosanol and penciclovir, can also be used to reduce symptoms such as itching, burning, and tingling.
Roseola and Fifth Disease
The viral diseases roseola and fifth disease are somewhat similar in terms of their presentation, but they are caused by different viruses. Roseola, sometimes called roseola infantum or exanthem subitum (“sudden rash”), is a mild viral infection usually caused by human herpesvirus-6 (HHV-6) and occasionally by HHV-7. It is spread via direct contact with the saliva or respiratory secretions of an infected individual, often through droplet aerosols. Roseola is very common in children, with symptoms including a runny nose, a sore throat, and a cough, along with (or followed by) a high fever (39.4 ºC). About three to five days after the fever subsides, a rash may begin to appear on the chest and abdomen. The rash, which does not cause discomfort, initially forms characteristic macules that are flat or papules that are firm and slightly raised; some macules or papules may be surrounded by a white ring. The rash may eventually spread to the neck and arms, and sometimes continues to spread to the face and legs. The diagnosis is generally made based upon observation of the symptoms. However, it is possible to perform serological tests to confirm the diagnosis. While treatment may be recommended to control the fever, the disease usually resolves without treatment within a week after the fever develops. For individuals at particular risk, such as those who are immunocompromised, the antiviral medication ganciclovir may be used.
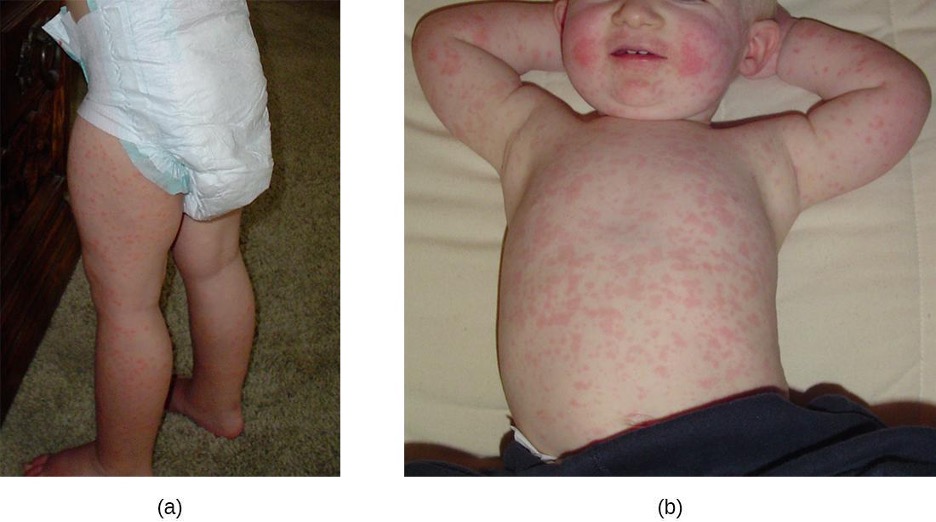
Fifth disease (also known as erythema infectiosum) is another common, highly contagious illness that causes a distinct rash that is critical to diagnosis. Fifth disease is caused by parvovirus B19, and is transmitted by contact with respiratory secretions from an infected individual. Infection is more common in children than adults. While approximately 20% of individuals will be asymptomatic during infection,[16] others will exhibit cold-like symptoms (headache, fever, and upset stomach) during the early stages when the illness is most infectious. Several days later, a distinct red facial rash appears, often called “slapped cheek” rash (figure 3.24). Within a few days, a second rash may appear on the arms, legs, chest, back, or buttocks. The rash may come and go for several weeks, but usually disappears within seven to twenty-one days, gradually becoming lacy in appearance as it recedes.
In children, the disease usually resolves on its own without medical treatment beyond symptom relief as needed. Adults may experience different and possibly more serious symptoms. Many adults with fifth disease do not develop any rash, but may experience joint pain and swelling that lasts several weeks or months. Immunocompromised individuals can develop severe anemia and may need blood transfusions or immune globulin injections. While the rash is the most important component of diagnosis (especially in children), the symptoms of fifth disease are not always consistent. Serological testing can be conducted for confirmation.
Viral Conjunctivitis
Like bacterial conjunctivitis, viral infections of the eye can cause inflammation of the conjunctiva and discharge from the eye. However, viral conjunctivitis tends to produce a discharge that is more watery than the thick discharge associated with bacterial conjunctivitis. The infection is contagious and can easily spread from one eye to the other or to other individuals through contact with eye discharge.
Viral conjunctivitis is commonly associated with colds caused by adenoviruses; however, other viruses can also cause conjunctivitis. If the causative agent is uncertain, eye discharge can be tested to aid in diagnosis. Antibiotic treatment of viral conjunctivitis is ineffective, and symptoms usually resolve without treatment within a week or two.
Herpes Keratitis
Herpes infections caused by HSV-1 can sometimes spread to the eye from other areas of the body, which may result in keratoconjunctivitis. This condition, generally called herpes keratitis or herpetic keratitis, affects the conjunctiva and cornea, causing irritation, excess tears, and sensitivity to light. Deep lesions in the cornea may eventually form, leading to blindness. Because keratitis can have numerous causes, laboratory testing is necessary to confirm the diagnosis when HSV-1 is suspected; once confirmed, antiviral medications may be prescribed.
| Disease | Pathogen | Signs and Symptoms | Tranmission | Antimicrobial Drugs |
|---|---|---|---|---|
| Fifth disease | Parvovirus B19 | May have initial cold-like symptoms; “slapped cheek” rash | Highly contagious via respiratory secretions of infected individuals | None |
| Herpes keratitis | Herpes simplex virus 1 (HSV-1) | Inflammation of conjunctiva and cornea; irritation, excess tears, sensitivity to light; lesions in cornea leading to blindness | Direct eye contact with discharge from herpes lesions elsewhere in the body or from another infected individual | Acyclovir, ganciclovir, famiclovir, valacyclovir |
| Oral herpes | Herpes simplex virus 1 (HSV-1) | May cause initial systemic symptoms; cold sores | Highly contagious via direct contact with infected individuals | Acyclovir, penciclovir, famiclovir, valacyclovir |
| Papillomas | Human papillomavirus (HPV) | Common warts, plantar warts, flat warts, filiform warts, and others | Contact with infected individuals | Topical salicylic acid, cantharidin |
| Roseola (roseola infantum, exanthem subitum) | Human herpesvirus 6 (HHV-6), human herpesvirus 7 (HHV-7) | Initial cold-like symptoms with high fever, followed by a macular rash three to five days later | Spread by viral and respiratory secretions of infected individuals | Typically none; ganciclovir for immunocompromised patients |
| Viral conjunctivitis | Adenoviruses and others | Inflammation of the conjunctiva; watery, nonpurulent discharge | Associated with common cold; contagious via contact with eye discharge | None |
Table 3.5: Viral infections of the skin and eyes
3.4 Mycoses of the Skin
Many fungal infections of the skin involve fungi that are found in the normal skin microbiota. Some of these fungi can cause infection when they gain entry through a wound; others mainly cause opportunistic infections in immunocompromised patients. Other fungal pathogens primarily cause infection in unusually moist environments that promote fungal growth; for example, sweaty shoes, communal showers, and locker rooms provide excellent breeding grounds that promote the growth and transmission of fungal pathogens.
Fungal infections, also called mycoses, can be divided into classes based on their invasiveness. Mycoses that cause superficial infections of the epidermis, hair, and nails, are called cutaneous mycoses. Mycoses that penetrate the epidermis and the dermis to infect deeper tissues are called subcutaneous mycoses. Mycoses that spread throughout the body are called systemic mycoses; common skin mycoses are summaries in table 3.7.
Tineas
A group of cutaneous mycoses called tineas are caused by dermatophytes, fungal molds that require keratin, a protein found in skin, hair, and nails, for growth. There are three genera of dermatophytes, all of which can cause cutaneous mycoses: Trichophyton, Epidermophyton, and Microsporum. Tineas on most areas of the body are generally called ringworm, but tineas in specific locations may have distinctive names and symptoms (see table 3.6 and figure 3.25). Keep in mind that these names—even though they are Latinized—refer to locations on the body, not causative organisms. Tineas can be caused by different dermatophytes in most areas of the body.
| Common tineas | Location |
|---|---|
| Tinea corporis (ringworm) | Body |
| Tinea capitis (ringworm) | Scalp |
| Tinea pedis (athlete’s foot) | Feet |
| Tinea barbae (barber’s itch) | Beard |
| Tinea cruris (jock itch) | Groin |
| Tinea unguium (onychomycosis) | Toenails, fingernails |
Table 3.6: Some common tineas and location on the body
Dermatophytes are commonly found in the environment and in soils and are frequently transferred to the skin via contact with other humans and animals. Fungal spores can also spread on hair. Many dermatophytes grow well in moist, dark environments. For example, tinea pedis (athlete’s foot) commonly spreads in public showers, and the causative fungi grow well in the dark, moist confines of sweaty shoes and socks. Likewise, tinea cruris (jock itch) often spreads in communal living environments and thrives in warm, moist undergarments.
Tineas on the body (tinea corporis) often produce lesions that grow radially and heal towards the center. This causes the formation of a red ring, leading to the misleading name of ringworm.
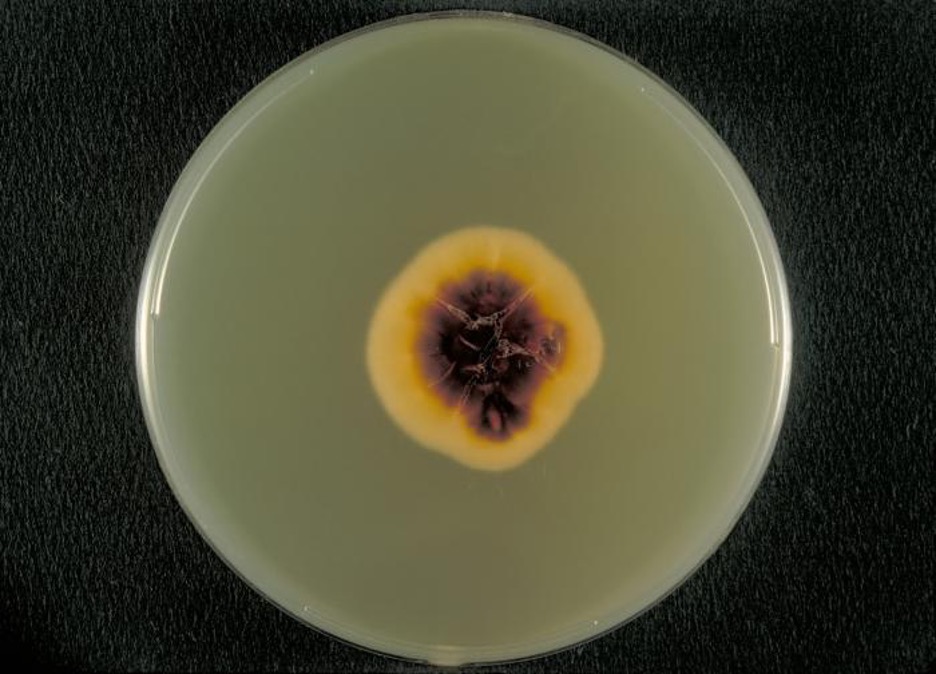
Several approaches may be used to diagnose tineas. A Wood’s lamp (also called a black lamp) with a wavelength of 365 nm is often used. When directed on a tinea, the ultraviolet light emitted from the Wood’s lamp causes the fungal elements (spores and hyphae) to fluoresce. Direct microscopic evaluation of specimens from skin scrapings, hair, or nails can also be used to detect fungi. Generally, these specimens are prepared in a wet mount using a potassium hydroxide solution (10%–20% aqueous KOH), which dissolves the keratin in hair, nails, and skin cells to allow for visualization of the hyphae and fungal spores. The specimens may be grown on Sabouraud dextrose CC (chloramphenicol/cycloheximide), a selective agar that supports dermatophyte growth while inhibiting the growth of bacteria and saprophytic fungi (figure 3.26). Macroscopic colony morphology is often used to initially identify the genus of the dermatophyte; identification can be further confirmed by visualizing the microscopic morphology using either a slide culture or a sticky tape prep stained with lactophenol cotton blue.
Various antifungal treatments can be effective against tineas. Allylamine ointments that include terbinafine are commonly used; miconazole and clotrimazole are also available for topical treatment, and griseofulvin is used orally.
Cutaneous Aspergillosis
Another cause of cutaneous mycoses is Aspergillus, a genus consisting of molds of many different species, some of which cause a condition called aspergillosis. Primary cutaneous aspergillosis, in which the infection begins in the skin, is rare but does occur. More common is secondary cutaneous aspergillosis, in which the infection begins in the respiratory system and disseminates systemically. Both primary and secondary cutaneous aspergillosis result in distinctive eschars that form at the site or sites of infection (figure 3.27). Pulmonary aspergillosis will be discussed more thoroughly in section 5.4.
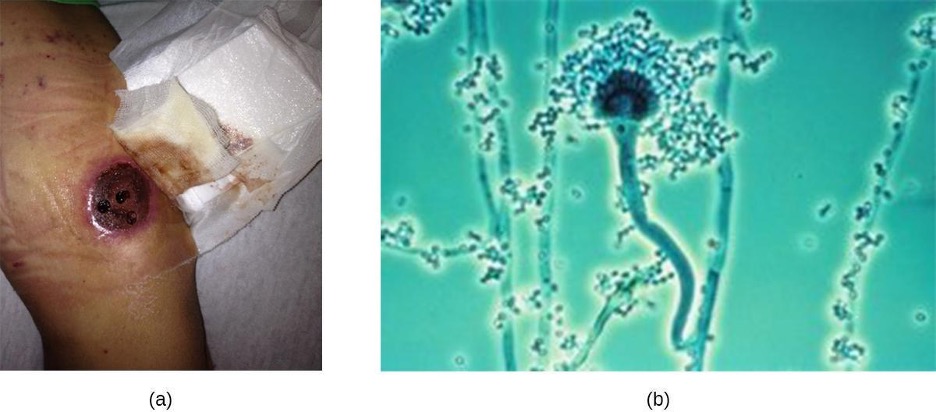
Primary cutaneous aspergillosis usually occurs at the site of an injury and is most often caused by Aspergillus fumigatus or Aspergillus flavus. It is usually reported in patients who have had an injury while working in an agricultural or outdoor environment. However, opportunistic infections can also occur in healthcare settings, often at the site of intravenous catheters, venipuncture wounds, or in association with burns, surgical wounds, or occlusive dressing. After candidiasis, aspergillosis is the second most common hospital-acquired fungal infection and often occurs in immunocompromised patients, who are more vulnerable to opportunistic infections.
Cutaneous aspergillosis is diagnosed using patient history, culturing, histopathology using a skin biopsy. Treatment involves the use of antifungal medications such as voriconazole (preferred for invasive aspergillosis), itraconazole, and amphotericin B if itraconazole is not effective. For immunosuppressed individuals or burn patients, medication may be used and surgical or immunotherapy treatments may be needed.
Candidiasis of the Skin and Nails
Candida albicans and other yeasts in the genus Candida can cause skin infections referred to as cutaneous candidiasis. Candida spp. are sometimes responsible for intertrigo, a general term for a rash that occurs in a skin fold, or other localized rashes on the skin. Candida can also infect the nails, causing them to become yellow and harden (figure 3.28).
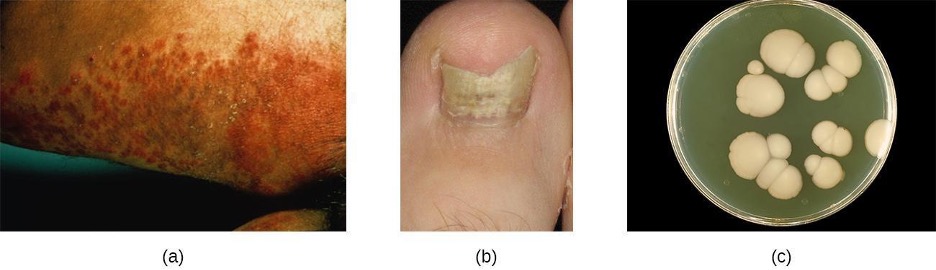
Candidiasis of the skin and nails is diagnosed through clinical observation and through culture, Gram stain, and KOH wet mounts. Susceptibility testing for antifungal agents can also be done. Cutaneous candidiasis can be treated with topical or systemic azole antifungal medications. Because candidiasis can become invasive, patients suffering from HIV/AIDS, cancer, or other conditions that compromise the immune system may benefit from preventive treatment. Azoles, such as clotrimazole, econazole, fluconazole, ketoconazole, and miconazole as well as the antifungal agents nystatin, terbinafine, and naftifine may be used for treatment. Long-term treatment with medications such as itraconazole or ketoconazole may be used for chronic infections. Repeat infections often occur, but this risk can be reduced by carefully following treatment recommendations, avoiding excessive moisture, maintaining good health, practicing good hygiene, and having appropriate clothing (including footwear).
Candida also causes infections in other parts of the body besides the skin. These include vaginal yeast infections (see section 7.5) and oral thrush (see section 4.2).
Sporotrichosis
Whereas cutaneous mycoses are superficial, subcutaneous mycoses can spread from the skin to deeper tissues. In temperate regions, the most common subcutaneous mycosis is a condition called sporotrichosis, caused by the fungus Sporothrix schenkii and commonly known as rose gardener’s disease, or rose thorn disease. Sporotrichosis is often contracted after working with soil, plants, or timber, as the fungus can gain entry through a small wound such as a thorn-prick or splinter. Sporotrichosis can generally be avoided by wearing gloves and protective clothing while gardening and promptly cleaning and disinfecting any wounds sustained during outdoor activities.
Sporothrix infections initially present as small ulcers in the skin, but the fungus can spread to the lymphatic system and sometimes beyond. When the infection spreads, nodules appear, become necrotic, and may ulcerate. As more lymph nodes become affected, abscesses and ulceration may develop over a larger area (often on one arm or hand). In severe cases, the infection may spread more widely throughout the body, although this is relatively uncommon.
Sporothrix infection can be diagnosed based upon histologic examination of the affected tissue. Its macroscopic morphology can be observed by culturing the mold on potato dextrose agar, and its microscopic morphology can be observed by staining a slide culture with lactophenol cotton blue. Treatment with itraconazole is generally recommended.
| Disease | Pathogen | Signs and Symptoms | Transmission | Antimicrobial Drugs |
|---|---|---|---|---|
| Aspergillosis (cutaneous) | Aspergillus fumigatus, Aspergillus flavus | Distinctive eschars at site(s) of infection | Entry via wound (primary cutaneous aspergillosis) or via the respiratory system (secondary cutaneous aspergillosis); commonly a hospital-acquired infection | Itraconazole, voriconazole, amphotericin B |
| Candidiasis (cutaneous) | Candida albicans | Intertrigo, localized rash, yellowing of nails | Opportunistic infections in immunocompromised patients | Azoles |
| Sporotrichosis (rose gardener’s disease) | Sporothrix schenkii | Subcutaneous ulcers and abscesses; may spread to a large area, e.g., hand or arm | Entry via thorn prick or other wound | Itraconazole |
| Tineas | Trichophyton spp., Epidermophyton spp., Microsporum spp. | Itchy, ring-like lesions (ringworm) at sites of infection | Contact with dermatophytic fungi, especially in warm, moist environments conducive to fungal growth | Terbinafine, miconazole, clotrimazole, griseofulvin |
Table 3.7: Mycoses of the skin
3.5 Protozoan and Helminthic Infections of the Skin and Eyes
Many parasitic protozoans and helminths use the skin or eyes as a portal of entry. Some may physically burrow into the skin or the mucosa of the eye; others breach the skin barrier by means of an insect bite. Still others take advantage of a wound to bypass the skin barrier and enter the body, much like other opportunistic pathogens. Although many parasites enter the body through the skin, in this chapter we will limit our discussion to those for which the skin or eyes are the primary site of infection. Parasites that enter through the skin but travel to a different site of infection will be covered in other chapters. In addition, we will limit our discussion to microscopic parasitic infections of the skin and eyes. Macroscopic parasites such as lice, scabies, mites, and ticks are beyond the scope of this text.
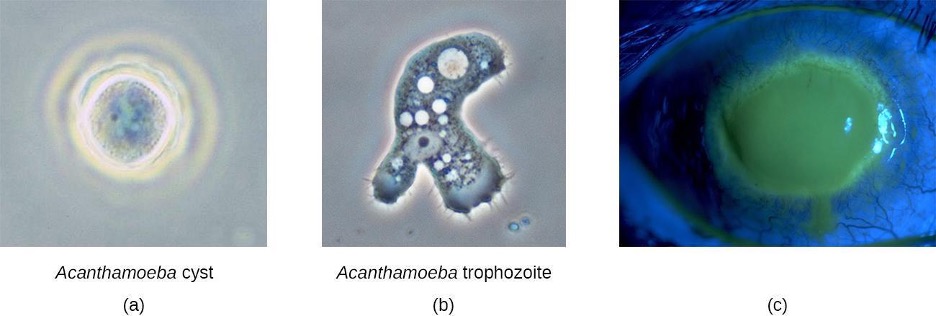
Acanthamoeba Infections
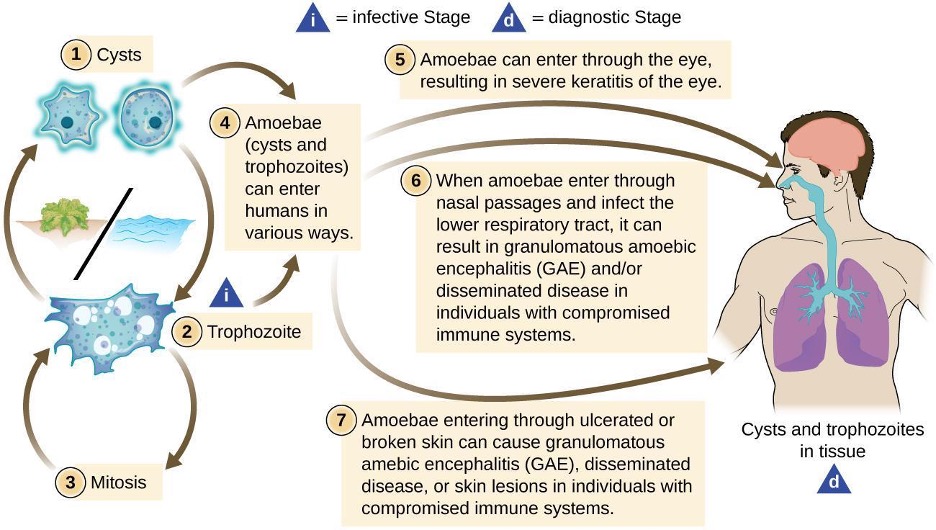
Acanthamoeba is a genus of free-living protozoan amoebae that are common in soils and unchlorinated bodies of freshwater. (This is one reason why some swimming pools are treated with chlorine.) The genus contains a few parasitic species, some of which can cause infections of the eyes, skin, and nervous system. Such infections can sometimes travel and affect other body systems. Skin infections may manifest as abscesses, ulcers, and nodules. When acanthamoebae infect the eye, causing inflammation of the cornea, the condition is called Acanthamoeba keratitis. Figure 3.29 illustrates the Acanthamoeba life cycle and various modes of infection.
While Acanthamoeba keratitis is initially mild, it can lead to severe corneal damage, vision impairment, or even blindness if left untreated. Similar to eye infections involving P. aeruginosa, Acanthamoeba poses a much greater risk to wearers of contact lenses because the amoeba can thrive in the space between contact lenses and the cornea. Prevention through proper contact lens care is important. Lenses should always be properly disinfected prior to use, and should never be worn while swimming or using a hot tub.
Acanthamoeba can also enter the body through other pathways, including skin wounds and the respiratory tract. It usually does not cause disease except in immunocompromised individuals; however, in rare cases, the infection can spread to the nervous system, resulting in a usually fatal condition called granulomatous amoebic encephalitis (GAE). Disseminated infections, lesions, and Acanthamoeba keratitis can be diagnosed by observing symptoms and examining patient samples under the microscope to view the parasite. Skin biopsies may be used.
Acanthamoeba keratitis is difficult to treat, and prompt treatment is necessary to prevent the condition from progressing. The condition generally requires three to four weeks of intensive treatment to resolve. Common treatments include topical antiseptics (e.g., polyhexamethylene biguanide, chlorhexidine, or both), sometimes with painkillers or corticosteroids (although the latter are controversial because they suppress the immune system, which can worsen the infection). Azoles are sometimes prescribed as well. Advanced cases of keratitis may require a corneal transplant to prevent blindness.
Loiasis
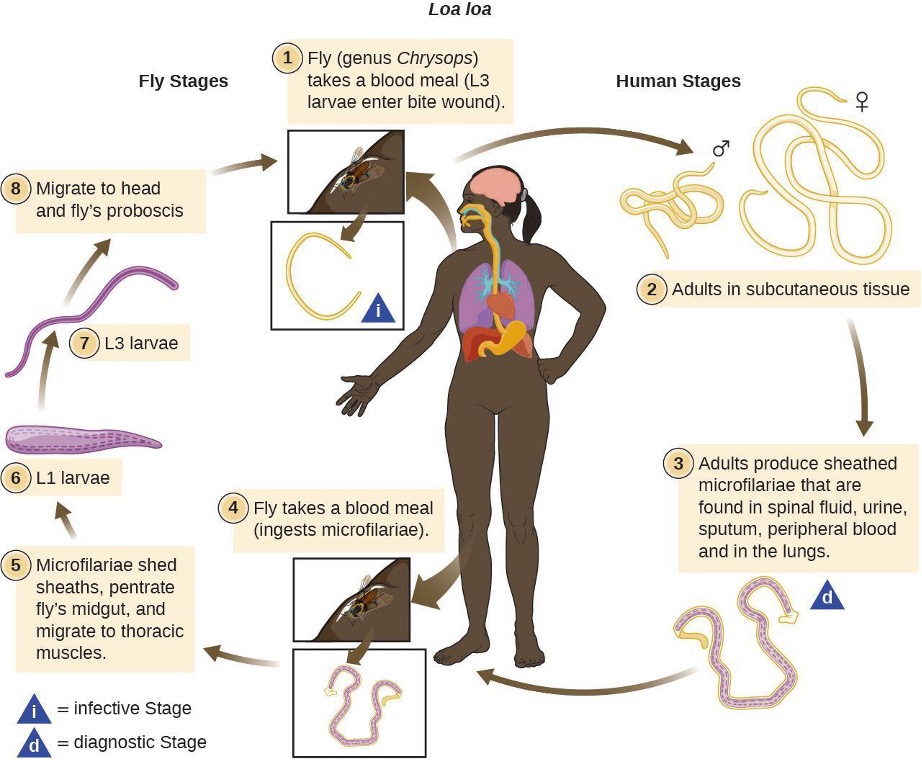
The helminth Loa loa, also known as the African eye worm, is a nematode that can cause loiasis, a disease endemic to West and Central Africa (figure 3.31 and table 3.8). The disease does not occur outside that region except when carried by travelers. There is evidence that individual genetic differences affect susceptibility to developing loiasis after infection by the Loa loa worm. Even in areas in which Loa loa worms are common, the disease is generally found in less than 30% of the population.[17] It has been suggested that travelers who spend time in the region may be somewhat more susceptible to developing symptoms than the native population, and the presentation of infection may differ.[18]
The parasite is spread by deer flies (genus Chrysops), which can ingest the larvae from an infected human via a blood meal (figure 3.31 and table 3.8). When the deer fly bites other humans, it deposits the larvae into their bloodstreams. After about five months in the human body, some larvae develop into adult worms, which can grow to several centimeters in length and live for years in the subcutaneous tissue of the host.
The name “eye worm” alludes to the visible migration of worms across the conjunctiva of the eye. Adult worms live in the subcutaneous tissues and can travel at about 1 cm per hour. They can often be observed when migrating through the eye, and sometimes under the skin; in fact, this is generally how the disease is diagnosed. It is also possible to test for antibodies, but the presence of antibodies does not necessarily indicate a current infection; it only means that the individual was exposed at some time. Some patients are asymptomatic, but in others the migrating worms can cause fever and areas of allergic inflammation known as Calabar swellings. Worms migrating through the conjunctiva can cause temporary eye pain and itching, but generally there is no lasting damage to the eye. Some patients experience a range of other symptoms, such as widespread itching, hives, and joint and muscle pain.
Worms can be surgically removed from the eye or the skin, but this treatment only relieves discomfort; it does not cure the infection, which involves many worms. The preferred treatment is diethylcarbamazine, but this medication produces severe side effects in some individuals, such as brain inflammation and possible death in patients with heavy infections. Albendazole is also sometimes used if diethylcarbamazine is not appropriate or not successful. If left untreated for many years, loiasis can damage the kidneys, heart, and lungs, though these symptoms are rare.
| Disease | Pathogen | Signs and Symptoms | Transmission | Antimicrobial Drugs |
|---|---|---|---|---|
| Acanthamoeba keratis | Acanthamoeba | Inflammation and damage to cornea; vision impairment or blindness | Exposure to pathogens in contaminated water or on contact lenses | Polyhexamethylene biguanide, chlorhexidine, azoles |
| Loiasis | Loa loa | Recurring fever and localized Calabar swelling, itching, and skin or eye pain during subcutaneous migration of worms | Larvae transmitted between humans by deerfly vector | Diethylcarbamazine, albendazole |
Table 3.8: Parasitic skin and eye infections
Summary
The following is a summary of the material covered throughout the chapter. It summarizes key aspects from each section and the pathogens included.
Bacterial Infections of the Skin and Eyes
- Staphylococcus and Streptococcus cause many different types of skin infections, a large number of which occur when bacteria breach the skin barrier through a cut or wound.
- S. aureus are frequently associated with purulent skin infections that manifest as folliculitis, furuncles, or carbuncles. S. aureus is also a leading cause of staphylococcal scalded skin syndrome (SSSS).
- S. aureus is generally drug resistant, and current MRSA strains are resistant to a wide range of antibiotics.
- Community-acquired and hospital-acquired staphyloccocal infections are an ongoing problem, because many people are asymptomatic carriers.
- Group A streptococci (GAS), S. pyogenes, is often responsible for cases of cellulitis, erysipelas, and erythema nosodum. GAS are also one of many possible causes of necrotizing fasciitis.
- P. aeruginosa is often responsible for infections of the skin and eyes, including wound and burn infections, hot tub rash, otitis externa, and bacterial keratitis.
- Acne is a common skin condition that can become more inflammatory when Propionibacterium acnes infects hair follicles and pores that are clogged with dead skin cells and sebum.
- Cutaneous anthrax occurs when Bacillus anthracis breaches the skin barrier. The infection results in a localized black eschar on skin. Anthrax can be fatal if B. anthracis spreads to the bloodstream.
- Common bacterial conjunctivitis is often caused by Haemophilus influenzae and usually resolves on its own in a few days. More serious forms of conjunctivitis include gonococcal ophthalmia neonatorum, inclusion conjunctivitis (chlamydial), and trachoma, all of which can lead to blindness if untreated.
- Keratitis is frequently caused by Staphylococcus epidermidis and/or Pseudomonas aeruginosa, especially among contact lens users and can lead to blindness.
- Biofilms complicate the treatment of wound and eye infections, because pathogens living in biofilms can be difficult to treat and eliminate.
| Disease | Pathogen | Signs and Symptoms | Transmission | Antimicrobial Drugs |
|---|---|---|---|---|
| Acne | Propionibacterium acnes | Comedones (whiteheads, blackheads); papules, pustules, nodules, or pseudocysts | Not transmissible; clogged pores become infected by normal skin microbiota (P. acnes) | Erythromycin, clindamycin |
| Anthrax (cutaneous) | Bacillus anthracis | Eschar at site of infection; may lead to septicemia and can be fatal | Entry of B. anthracis endospores through cut or abrasion | Penicillin, erythromycin, or tetracycline |
| Cellulitis | Streptococcus pyogenes | Localized inflammation of dermis and hypodermis; skin red, warm, and painful to the touch | Entry of S. pyogenes through cut or abrasion | Oral or intravenous antibiotics (e.g., penicillin) |
| Erysipelas | S. pyogenes | Inflamed, swollen patch of skin, often on face; may be suppurative | Entry of S. pyogenes through cut or abrasion | Oral or intravenous antibiotics (e.g., penicillin) |
| Erythema nodosum | S. pyogenes | Small red nodules, often on shins | Associated with other streptococcal infection | None or anti-inflammatory drugs for severe cases |
| Impetigo | Staphylococcus aureus, S. pyogenes | Vesicles, pustules, and sometimes bullae around nose and mouth | Highly contagious, especially via contact | Topical or oral antibiotics. |
| Necrotizing fasciitis | S. pyogenes, Klebsiella, Clostridium, others | Infection of fascia and rapidly spreading tissue death; can lead to septic shock and death | Entry of bacteria through cut or abrasion | Intravenous broad-spectrum antibiotics |
| Otitis externa | Pseudomonas aeruginosa | Itching, redness, discomfort of ear canal, progressing to fever, pain, swelling | P. aeruginosa enters ear canal via pool or other water | Acidic ear drops with antibiotics, antifungals, steroids |
| Staphylococcal scalded skin syndrome (SSSS) | S. aureus | Erythema and severe peeling of skin | Infection of skin and mucous membranes, especially in children | Intravenous antibiotics, fluid therapy |
| Wound infections | P. aeruginosa, others | Formation of biofilm in or on wound | Exposure of wound to microbes in environment; poor wound hygiene | Polymyxin B, gentamicin, fluoroquinolones, topical anti-biofilm agents. |
Table 3.9: Bacterial infections of the skin
| Disease | Pathogen | Signs and Symptoms | Transmission | Antimicrobial Drugs |
|---|---|---|---|---|
| Acute bacterial conjunctivitis | Haemophilus influenza | Inflammation of conjunctiva with purulent discharge | Exposure to secretions from infected individuals | Broad-spectrum topical antibiotics |
| Bacterial keratitis | Staphylococcus epidermidis, Pseudomonas aeruginosa | Redness and irritation of eye, blurred vision, sensitivity to light; progressive corneal scarring, which can lead to blindness | Exposure to pathogens on contaminated contact lenses | Antibiotic eye drops (e.g., with fluoroquinolones) |
| Neonatal conjunctivitis | Chlamydia trachomatis, Neisseria gonorrhoeae | Inflammation of conjunctiva, purulent discharge, scarring and perforation of cornea; may lead to blindness | Neonate exposed to pathogens in birth canal of a pregnant person with chlamydia or gonorrhea | Erythromycin |
| Trachoma (granular conjunctivitis) | C. trachomatis | Chronic conjunctivitis, trichiasis, scarring, blindness | Contact with infected individuals or contaminated fomites; transmission by eye-seeking flies | Azithromycin |
Table 3.10: Bacterial infections of the eyes
Viral Infections of the Skin and Eyes
- Papillomas (warts) are caused by human papillomaviruses.
- Herpes simplex virus (especially HSV-1) mainly causes oral herpes, but lesions can appear on other areas of the skin and mucous membranes.
- Roseola and fifth disease are common viral illnesses that cause skin rashes; roseola is caused by HHV-6 and HHV-7 while fifth disease is caused by parvovirus 19.
- Viral conjunctivitis is often caused by adenoviruses and may be associated with the common cold. Herpes keratitis is caused by herpesviruses that spread to the eye.
| Disease | Pathogen | Signs and Symptoms | Tranmission | Antimicrobial Drugs |
|---|---|---|---|---|
| Fifth disease | Parvovirus B19 | May have initial cold-like symptoms; “slapped cheek” rash | Highly contagious via respiratory secretions of infected individuals | None |
| Herpes keratitis | Herpes simplex virus 1 (HSV-1) | Inflammation of conjunctiva and cornea; irritation, excess tears, sensitivity to light; lesions in cornea leading to blindness | Direct eye contact with discharge from herpes lesions elsewhere in the body or from another infected individual | Acyclovir, ganciclovir, famiclovir, valacyclovir |
| Oral herpes | Herpes simplex virus 1 (HSV-1) | May cause initial systemic symptoms; cold sores | Highly contagious via direct contact with infected individuals | Acyclovir, penciclovir, famiclovir, valacyclovir |
| Papillomas | Human papillomavirus (HPV) | Common warts, plantar warts, flat warts, filiform warts, and others | Contact with infected individuals | Topical salicylic acid, cantharidin |
| Roseola (roseola infantum, exanthem subitum) | Human herpesvirus 6 (HHV-6), human herpesvirus 7 (HHV-7) | Initial cold-like symptoms with high fever, followed by a macular rash three to five days later | Spread by viral and respiratory secretions of infected individuals | Typically none; ganciclovir for immunocompromised patients |
| Viral conjunctivitis | Adenoviruses and others | Inflammation of the conjunctiva; watery, nonpurulent discharge | Associated with common cold; contagious via contact with eye discharge | None |
Table 3.11: Viral infections of the skin and eyes
Mycoses of the Skin
- Mycoses can be cutaneous, subcutaneous, or systemic.
- Common cutaneous mycoses include tineas caused by dermatophytes of the genera Trichophyton, Epidermophyton, and Microsporum. Tinea corporis is called ringworm. Tineas on other parts of the body have names associated with the affected body part.
- Aspergillosis is a fungal disease caused by molds of the genus Aspergillus. Primary cutaneous aspergillosis enters through a break in the skin, such as the site of an injury or a surgical wound; it is a common hospital-acquired infection. In secondary cutaneous aspergillosis, the fungus enters via the respiratory system and disseminates systemically, manifesting in lesions on the skin.
- The most common subcutaneous mycosis is sporotrichosis (rose gardener’s disease), caused by Sporothrix schenkii.
- Yeasts of the genus Candida can cause opportunistic infections of the skin called candidiasis, producing intertrigo, localized rashes, or yellowing of the nails.
| Disease | Pathogen | Signs and Symptoms | Transmission | Antimicrobial Drugs |
|---|---|---|---|---|
| Aspergillosis (cutaneous) | Aspergillus fumigatus, Aspergillus flavus | Distinctive eschars at site(s) of infection | Entry via wound (primary cutaneous aspergillosis) or via the respiratory system (secondary cutaneous aspergillosis); commonly a hospital-acquired infection | Itraconazole, voriconazole, amphotericin B |
| Candidiasis (cutaneous) | Candida albicans | Intertrigo, localized rash, yellowing of nails | Opportunistic infections in immunocompromised patients | Azoles |
| Sporotrichosis (rose gardener’s disease) | Sporothrix schenkii | Subcutaneous ulcers and abscesses; may spread to a large area, e.g., hand or arm | Entry via thorn prick or other wound | Itraconazole |
| Tineas | Trichophyton spp., Epidermophyton spp., Microsporum spp. | Itchy, ring-like lesions (ringworm) at sites of infection | Contact with dermatophytic fungi, especially in warm, moist environments conducive to fungal growth | Terbinafine, miconazole, clotrimazole, griseofulvin |
Table 3.12: Mycoses of the skin
Protozoan and Helminthic Infections of the Skin and Eyes
- The protozoan Acanthamoeba and the helminth Loa loa are two parasites that can breach the skin barrier, leading to infections of the skin and eyes.
- Acanthamoeba keratitis is a parasitic infection of the eye that often results from improper disinfection of contact lenses or swimming while wearing contact lenses.
- Loiasis, or eye worm, is a disease endemic to Africa that is caused by parasitic worms that infect the subcutaneous tissue of the skin and eyes. It is transmitted by deer fly vectors.
| Disease | Pathogen | Signs and Symptoms | Transmission | Antimicrobial Drugs |
|---|---|---|---|---|
| Acanthamoeba keratis | Acanthamoeba | Inflammation and damage to cornea; vision impairment or blindness | Exposure to pathogens in contaminated water or on contact lenses | Polyhexamethylene biguanide, chlorhexidine, azoles |
| Loiasis | Loa loa | Recurring fever and localized Calabar swelling, itching, and skin or eye pain during subcutaneous migration of worms | Larvae transmitted between humans by deerfly vector | Diethylcarbamazine, albendazole |
Table 3.13: Parasitic skin and eye infections
Figure Descriptions
Figure 3.1: a) A micrograph of a large light pink region labeled dermis, a thinner dark pink region on top of that labeled epidermis, and a thin region of clear cells. The division between the dermis and epidermis is wavy; with areas where one projects into the other. B) A diagram of skin. The top layer is dark and is labeled epidermis. The next layer is lighter and much thicker; this is the dermis. Inside the dermis are vase-shaped hair follicles with hairs projecting out of the skin. Next to the hair follicle is a smaller vase-shape labeled sebaceous gland; this empties into the space of the hair follicle. There are also coiled shapes labeled receptor and a variety of long tubes labeled: nerve, lymph vessel and blood vessels. A coiled blob is labeled sweat gland; this leads to a tube that opens at the surface called a sweat pore. Below the dermis is a yellow bubbly-looking layer labeled fatty tissue; this is the hypodermis.
Figure 3.2: A diagram showing different regions of the body. Each region has a pie chart that shows which bacteria are most prevalent. The most common bacterium in each region: Glabella (corynebacterineae), Alar Crease (propionibacterineae), External auditory canal (propionibacterineae), Nare (other actinobacteria), manubrium (propionibacterineae), Axillary vault (proteobacteria), antecubital fossa (proteobacteria), Volar forearm (proteobacteria), interdigital web space (proteobacteria), hypothenar palm (proteobacteria), inguinal crease (corynebacterineae), umbilicus (corynebacterineae), toe web space (corynebacterineae, , propionibacterineae, and staphylococcaceae), reticular crease (propionibacterineae), occiput (staphylococcaceae, back (propionibacterineae), buttock (proteobacteria), gluteal crease (corynebacterineae), popliteal fossa (staphylococcaceae), plantar heel (staphylococcaceae). Second part of the image shows that different subjects have different bacterial percentages and that these percentages change over time.
Figure 3.3: a) Acne (labeled whitehead) on a person’s cheek. B) A drawing of skin with a yellow bubble labeled pus. This is below a raised region on the skin.
Figure 3.4: A table labeled types of skin lesions. Crust is shown as a raised region on the surface of the skin. Cyst is shown as a large white sphere in the upper layers of the skin. Macule is shown as a dark mark on the surface. Papule is shown as a raised bubble on the surface. Pusture is shown as a large yellow sphere in the upper layers of the skin. Ulcer is a large cavity in the skin. Vesicle is a small blue bubble in the upper regions of the skin. Wheal is a small blue bubble on the surface of the skin.
Figure 3.5: Diagram of an eye. Above the eye is the lacrimal gland. At the point nearest the nose is the punctums and tubes leading to the lacrimal sac and nasolacrimal duct.
Figure 3.6: A cross section of the eye. The large spherical center is the vitreous humor. The layer surrounding this is the retina. A projection out of the back of the eye is the optic nerve. A region on the retina just above the optic nerve is the fovea. At the front of the eye is the lens. In front of this is a space labeled pupil. The colored region around the pupil is the iris. The cornea is the covering in front of the iris and pupil. The conjunctiva is a mucous membrane on the eye.
Figure 3.7: a) Photo of an eyelid being pulled back to show a red eye. B) A photo of inflamed eyelids. C) A photo of an eye with a cloudy cornea.
Figure 3.8: a) An agar plate with 2 regions of growth. One region has a yellow background, the other has a pink background. B) A micrograph of clusters of round cells. Each cell is just under 1 µm in diameter.
Figure 3.9: a) a photo of a small inflamed region with a white center. b) A large lesion with white and red.
Figure 3.10: Skin peeling off a hand.
Figure 3.11: Red bumps on the skin above the mouth.
Figure 3.12: A micrograph of small purple circles chained together in clumps.
Figure 3.13: a) a red rash. B) swollen, red regions on the cheeks and nose. C) red lumps on the skin.
Figure 3.14: a) skin with large black, grey and red regions. b) Cut skin in surgery.
Figure 3.15: a) photo of acne b) photo of swollen ear.
Figure 3.16: a) A red plate with white colonies. B) A clear plate with green colonies; the green extends past the colony. C) a dark plate with glowing colonies.
Figure 3.17: a) diagram of blackhead formation. A normal pore in the skin becomes filled with material forming a whitehead. Darker material forms a blackhead. B) blackheads on a nose.
Figure 3.18: a) A black nodule on skin. b) A red plate with grey colonies.
Figure 3.19: Eye with yellow discharge.
Figure 3.20: Swollen eyes with discharge.
Figure 3.21: a) eye with turned in eyelids. B) photo of eye surgery.
Figure 3.22: a) photo of warts on a toe. B) photo of wart on an eye.
Figure 3.23: Photo of cold sore on a lip.
Figure 3.24: a) photo of red patches on an infant’s legs. B) photo of red spots on a child’s trunk.
Figure 3.25: a) large red bumps on a cheek and neck. B) white crusty skin on a foot. C) an orange ring on skin.
Figure 3.26: A photo of a large black, fuzzy colony.
Figure 3.27: a) photo of a large, round, dark area on their leg. B) many thick strands and small dots. One of the strands ends in a sphere with long chains of dots around the top part of the structure.
Figure 3.28: A) a dark, lumpy rash. B) a broken, yellow nail. C) large, white, fuzzy colonies on a plate.
Figure 3.29: Live cycle of Acanthamoeba. In the water the cyst becomes a trophozoite. This then undergoes mitosis to form more trophozoites. Trophozoites can also become cysts. The amebae (cysts and trophozoites) can enter humans in various ways. Amoebae can enter through the eye, resulting in severe keratitis of the eye. When amoebae enter through nasal passages and infect the lower respiratory tract, it can result in granulomatous amebic encephalitis (GAE) and/or disseminated disease in individuals with compromised immune systems. Amoebae entering through ulcerated or broken skin can cause granulomatous amebic encephalitis (GAE), disseminated disease, or skin lesions in individuals with compromised immune systems.
Figure 3.30: a) an acanthamoeba cyst is shown. b) an acanthamoeba trophozoite micrograph is shown. c) a photo of an eye with a fluorescent cornea is shown.
Figure 3.31: The first part of the image is a photograph of an eye with a visible worm inside of it and a photo of a close-up of the worm. The second image is an illustrated chart showing the Life cycle of Lao lao. Fly (genus Chrysops) takes a blood meal (L3 larvae enter the bite wound). Adults grow into long worms in the subcutaneous tissue. Adults produce sheathed microfilariae that are found in spinal fluid, urine, sputum, peripheral blood, and in the lungs. Another fly take a blood meal and ingests microfilariae. The microfilariae shed sheaths, penetrate fly’s midgut, and migrate to thoracic muscles. The L1 larvae forms and becomes an L3 larvae which migrates to the head and fly’s proboscis. The fly is now ready to infect another person.
Figure References
Figure 3.1: A micrograph of a section through human skin shows the epidermis and dermis. Left: Modification of work by Kibad. Public Domain. https://commons.wikimedia.org/wiki/File:Normal_Epidermis_and_Dermis_with_Intradermal_Nevus_10x.JPG. Right: Modification of work by National Cancer Institute. Public Domain. https://commons.wikimedia.org/wiki/File:Anatomy_The_Skin_-_NCI_Visuals_Online.jpg
Figure 3.2: The normal microbiota varies on different regions of the skin, especially in dry versus moist areas. Modification of work by National Human Genome Research Institute. Public domain.
Figure 3.3: Acne is a bacterial infection of the skin that manifests as a rash of inflamed hair follicles (folliculitis). Left: by Modification of work by Diariodaj. Public Domain. https://commons.wikimedia.org/wiki/File:Teenager-with-acne.jpg. Right: Modification of work by (c) BruceBlaus. Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. https://commons.wikimedia.org/wiki/File:Blausen_0007_Abscess.png
Figure 3.4: Numerous causes can lead to skin lesions of various types, some of which are very similar in appearance. Modification of work (c) Bruce Blaus. CC BY 4.0.
Figure 3.5: The lacrimal apparatus includes the structures of the eye associated with tear production and drainage. Modification of work (c) Evidence Based Medical Educator Inc./YouTube. CC BY 4.0.
Figure 3.6: Some microbes live on the conjunctiva of the human eye, but the vitreous humor is sterile. (c) Rice University. OpenStax Microbiology. CC BY 4.0. https://openstax.org/details/books/microbiology.
Figure 3.7: Left: Conjunctivitis is inflammation of the conjunctiva. modification of Figure 4 from Lopez-Prats MJ, Sanz Marco E, Hidalgo-Mora JJ, Garcia-Delpech S, Diaz-Llopis M. 2010. “Bleeding Follicular Conjunctivitis due to Influenza H1N1 Virus”. Journal of Ophthalmology. https://doi.org/10.1155/2010/423672. CC BY 3.0. Middle and Right: Modification of work by Centers for Disease Control and Prevention. Public Domain.
Figure 3.8: (a) A mannitol salt agar plate is used to distinguish different species of staphylococci. Left: modification of work by “ScienceProfOnline”/YouTube. Public domain. Right: modification of work by Centers for Disease Control and Prevention. Public domain.
Figure 3.9: Furuncles (boils) and carbuncles are infections of the skin often caused by Staphylococcus bacteria. Left: Public Health Image Library / CDC; Public Domain. Right: modification of work by Drvgaikwad. CC BY SA 3.0 Unported. https://commons.wikimedia.org/wiki/File:Carbuncle_on_buttok.JPG
Figure 3.10: A newborn with staphylococcal scalded skin syndrome (SSSS), which results in large regions of peeling, dead skin. Modification of Figure 2 in Jeyakumari, D; Gopal, R; Eswaran, M1; MaheshKumar, C1. Staphylococcal Scalded Skin Syndrome in a Newborn. Journal of Global Infectious Diseases 1(1):p 45-47, Jan–Jun 2009. | DOI: 10.4103/0974-777X.52981. CC BY 4.0.
Figure 3.11 Impetigo is characterized by vesicles, pustules, or bullae that rupture, producing encrusted sores. Modification of work by FDA. Public domain.
Figure 3.12: Streptococcus pyogenes forms chains of cocci. Modification of work by Centers for Disease Control and Prevention. Public domain.
Figure 3.13: S. pyogenes can cause a variety of skin conditions once it breaches the skin barrier through a cut or wound. Left: (c) modification Figure 1 in Bassukas, I.D., Gaitanis, G., Zioga, A. et al. Febrile “migrating” eosinophilic cellulitis with hepatosplenomegaly: adult toxocariasis – a case report. Cases Journal 1, 356 (2008). https://doi.org/10.1186/1757-1626-1-356. CC BY 2.0. Middle: Modification of work by Centers for Disease Control and Prevention. Public Domain. Right: Modification of Figure 1 in Dean, C., Crow, W. Painful red nodules in female patient with recent travel history: a case report. Cases Journal 2, 8248 (2009). https://doi.org/10.4076/1757-1626-2-8248. CC BY 2.0.
Figure 3.14: The left leg of this patient shows the clinical features of necrotizing fasciitis. Figures 1 and 2 in Smuszkiewicz, P., Trojanowska, I. & Tomczak, H. Late diagnosed necrotizing fasciitis as a cause of multiorgan dysfunction syndrome: A case report. Cases Journal 1, 125 (2008). https://doi.org/10.1186/1757-1626-1-125. CC BY 2.0.
Figure 3.15: Hot tub folliculitis presents as an itchy red rash. Left: Lsupellmel. Public Domain. https://commons.wikimedia.org/wiki/File:HotTubFolliculitis.jpg. Right: Modification of work (c) Klaus D. Peter. CC BY 3.0 Germany. https://commons.wikimedia.org/wiki/File:Otitis_externa.jpg
Figure 3.16: These P. aeruginosa colonies are growing on xylose lysine sodium deoxycholate (XLD) agar. Left: modification of work by Centers for Disease Control and Prevention. Public Domain. Middle and Right: Public Domain.
Figure 3.17: Acne is characterized by whitehead and blackhead comedones that result from clogged hair follicles. Left: Modification of work (c) Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. CC BY 3.0 Unported. https://commons.wikimedia.org/wiki/File:Blausen_0811_SkinPores.png. Right: By Elecbullet. Public Domain. https://commons.wikimedia.org/wiki/File:Blackheads.JPG
Figure 3.18: (a) Cutaneous anthrax is an infection of the skin by B. anthracis, which produces tissue-damaging exotoxins. Modification of work by Centers for Disease Control and Prevention. Public domain.
Figure 3.19: Acute, purulent, bacterial conjunctivitis causes swelling and redness in the conjunctiva, the membrane lining the whites of the eyes and the inner eyelids. (c) “Tanalai. CC BY 3.0. https://commons.wikimedia.org/wiki/File:Swollen_eye_with_conjunctivitis.jpg
Figure 3.20: A newborn suffering from gonococcal ophthalmia neonatorum. Centers for Disease Control and Prevention. Public domain.
Figure 3.21: If trachoma is not treated early with antibiotics, scarring on the eyelid can lead to trichiasis, a condition in which the eyelashes turn inward. Left: (c) Rice University. OpenStax Microbiology. CC BY 4.0. https://openstax.org/details/books/microbiology. Right: Modification of work (c) Otis Historical Archives National Museum of Health & Medicine. CC BY 2.0. https://commons.wikimedia.org/wiki/File:Entropion_and_trichiasis_secondary_to_trachoma_A44-652-11.jpg
Figure 3.22: Warts can vary in shape and in location. Credit a: Raimar. Public Domain. https://commons.wikimedia.org/wiki/File:Dornwarzen.jpg. Credit b: Schweintechnik. Public Domain. https://commons.wikimedia.org/wiki/File:Wart_filiform_eyelid.jpg
Figure 3.23: This cold sore was caused by HSV-1. Centers for Disease Control and Prevention. Public domain.
Figure 3.24: Roseola. Credit a: Modified from work of M Davis. Public Domain. https://commons.wikimedia.org/wiki/File:Roseola_on_a_21-month-old_girl.jpg. Credit b: Modified from work of Andrew Kerr. Public Domain. https://en.wikipedia.org/wiki/File:Fifth_disease.jpg
Figure 3.25: Tineas are superficial cutaneous mycoses and are common. Left, Right: Modification of work by Centers for Disease Control and Prevention. Public Domain. Middle: Figure 1 from Al Hasan, M., Fitzgerald, S.M., Saoudian, M. et al. Dermatology for the practicing allergist: Tinea pedis and its complications. Clin Mol Allergy 2, 5 (2004). Fair Use. https://doi.org/10.1186/1476-7961-2-5
Figure 3.26: To diagnose tineas, the dermatophytes may be grown on a Sabouraud dextrose CC agar plate. Centers for Disease Control and Prevention. Public domain.
Figure 3.27 Eschar on a patient with secondary cutaneous aspergillosis. Left: Figure 1 in Santiago, Mónica, Martinez, José Hernán, Palermo, Coromoto, Figueroa, Carlos, Torres, Oberto, Trinidad, Rafael, Gonzalez, Eva, Miranda, Maria de Lourdes, Garcia, Miosotis, Villamarzo, Guillermo, Rapidly Growing Thyroid Mass in an Immunocompromised Young Male Adult, Case Reports in Endocrinology, 2013, 290843, 4 pages, 2013. https://doi.org/10.1155/2013/290843. CC BY 3.0. Right: Modification of work by U.S. Department of Health and Human Services. Public Domain.
Figure 3.28: This red, itchy rash is the result of cutaneous candidiasis, an opportunistic infection of the skin caused by the yeast Candida albicans. Left: modification of work by U.S. Department of Veterans Affairs. Public Domain. Middle: Medguy. https://commons.wikimedia.org/wiki/File:Toefungus.jpg. Public Domain. Right: modification of work by Centers for Disease Control and Prevention.
Figure 3.29: Acanthamoeba spp. are waterborne parasites very common in unchlorinated aqueous environments. Modification of work by Centers for Disease Control and Prevention. Public domain.
Figure 3.30: An Acanthamoeba cyst. An Acanthamoeba trophozoite. Left: modification of work by Centers for Disease Control and Prevention. Public Domain. Middle, Right: Modification Figure 1, 3 from Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. https://pmc.ncbi.nlm.nih.gov/articles/PMC4330640. CC BY 4.0.
Figure 3.31: This Loa loa worm, measuring about 55 mm long, was extracted from the conjunctiva of a patient with loiasis. Modification of work by Centers for Disease Control and Prevention. Public domain.
Text References
- Belkaid, Y., and J.A. Segre. “Dialogue Between Skin Microbiota and Immunity,” Science 346 (2014) 6212:954–959. ↵
- Foulongne, Vincent, et al. “Human Skin Microbiota: High Diversity of DNA Viruses Identified on the Human Skin by High Throughput Sequencing.” PLoS ONE (2012) 7(6): e38499. doi: 10.1371/journal.pone.0038499. ↵
- Robinson, C.M., and J.K. Pfeiffer. “Viruses and the Microbiota.” Annual Review of Virology (2014) 1:55–59. doi: 10.1146/annurev-virology-031413-085550. ↵
- Abelson, M.B., Lane, K., and Slocum, C.. “The Secrets of Ocular Microbiomes.” Review of Ophthalmology (June 8, 2015). http://www.reviewofophthalmology.com/content/t/ocular_disease/c/55178. Accessed Sept 14, 2016. ↵
- Shaikh-Lesko, R. “Visualizing the Ocular Microbiome.” The Scientist (May 12, 2014). http://www.the-scientist.com/?articles.view/articleNo/39945/title/Visualizing-the-Ocular-Microbiome. Accessed Sept 14, 2016. ↵
- Starr, C.R. and Engelberg N.C. “Role of Hyaluronidase in Subcutaneous Spread and Growth of Group A Streptococcus.” Infection and Immunity (2006) (7:1): 40–48. doi: 10.1128/IAI.74.1.40-48.2006. ↵
- Nuwayhid, Z.B., Aronoff, D.M., and Mulla, Z.D.. “Blunt Trauma as a Risk Factor for Group A Streptococcal Necrotizing Fasciitis.” Annals of Epidemiology (2007) 17:878–881. ↵
- Shadomy, S.V., Traxler, R.M., and Marston, C.K. “Infectious Diseases Related to Travel: Anthrax.” Centers for Disease Control and Prevention. (2015) http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/anthrax. Accessed Sept 14, 2016. ↵
- US FDA. “Anthrax.” 2015. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ucm061751.htm. Accessed Sept 14, 2016. ↵
- Berger, T., Kassirer, M., and Aran, A.A.. “Injectional Anthrax—New Presentation of an Old Disease.” Euro Surveillance 19 (2014) 32. http://www.ncbi.nlm.nih.gov/pubmed/25139073. Accessed Sept 14, 2016. ↵
- United Nations Office at Geneva. “What Are Biological and Toxin Weapons?” http://www.unog.ch/80256EE600585943/%28httpPages%29/29B727532FECBE96C12571860035A6DB?. Accessed Sept 14, 2016. ↵
- Federal Bureau of Investigation. “Famous Cases and Criminals: Amerithrax or Anthrax Investigation.” https://www.fbi.gov/history/famous-cases/amerithrax-or-anthrax-investigation. Accessed Sept 14, 2016. ↵
- Centers for Disease Control and Prevention. “Anthrax: Medical Care: Prevention: Antibiotics.” http://www.cdc.gov/anthrax/medical-care/prevention.html. Accessed Sept 14, 2016. ↵
- Emergent Biosolutions. AVA (BioThrax) vaccine package insert (Draft). Nov 2015. http://www.fda.gov/downloads/biologicsbloodvaccines/bloodbloodproducts/approvedproducts/licensedproductsblas/ucm074923.pdf. ↵
- Wald, A., and Corey, L. “Persistence in the Population: Epidemiology, Transmission.” In: A. Arvin, G. Campadelli-Fiume, E. Mocarski et al. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press, 2007. http://www.ncbi.nlm.nih.gov/books/NBK47447/. Accessed Sept 14, 2016. ↵
- Centers for Disease Control and Prevention. “Fifth Disease.” http://www.cdc.gov/parvovirusb19/fifth-disease.html. Accessed Sept 14, 2016. ↵
- Garcia, A.. et al. “Genetic Epidemiology of Host Predisposition Microfilaraemia in Human Loiasis.” Tropical Medicine and International Health 4 (1999) 8:565–74. http://www.ncbi.nlm.nih.gov/pubmed/10499080. Accessed Sept 14, 2016. ↵
- Spinello, A., et al. “Imported Loa loa Filariasis: Three Cases and a Review of Cases Reported in Non-Endemic Countries in the Past 25 Years.” International Journal of Infectious Disease 16 (2012) 9: e649–e662. DOI: http://dx.doi.org/10.1016/j.ijid.2012.05.1023. ↵

